Sex-specific differences in hyperoxic lung injury in mice: role of cytochrome P450 (CYP)1A
- PMID: 25703676
- PMCID: PMC4406849
- DOI: 10.1016/j.tox.2015.01.019
Sex-specific differences in hyperoxic lung injury in mice: role of cytochrome P450 (CYP)1A
Abstract
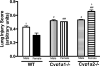
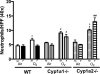
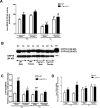
Sex-specific differences in pulmonary morbidity in adults and preterm infants are well documented. Hyperoxia contributes to lung injury in experimental animals and humans. Cytochrome P450 (CYP) 1A enzymes have been shown to play a mechanistic role in hyperoxic lung injury (HLI) in animal models. Whether CYP1A enzymes contribute to gender-specific differences in relation to HLI is unknown. In this investigation, we tested the hypothesis that mice will display gender-specific differences in HLI, and that this phenomenon will be altered in mice lacking the genes for Cyp1a1 or 1a2. Eight week-old male and female wild type (WT) (C57BL/6J) mice, Cyp1a1-/-, and Cyp1a2-/- mice were exposed to 72h of hyperoxia (FiO2>0.95). Lung injury and inflammation were assessed and pulmonary and hepatic CYP1A1 and CYP1A2 levels were quantified at the enzyme activity, protein and mRNA level. Upon exposure to hyperoxia, liver and lung microsomal proteins showed higher pulmonary CYP1A1 (apoprotein level and activity) in WT females compared to WT males and a greater induction in hepatic CYP1A2 mRNA levels and activity in WT females after hyperoxia exposure. The gender based female advantage was lost or reversed in Cyp1a1-/- and Cyp1a2-/- mice. These findings suggest an important role for CYP1A enzymes in the gender-specific modulation of hyperoxic lung injury.
Keywords: CYP1A; Hyperoxia; Lung injury; Sex-specific.
Copyright © 2015 Elsevier Ireland Ltd. All rights reserved.
Figures




Similar articles
-
Role of Cytochrome P450 (CYP)1A in Hyperoxic Lung Injury: Analysis of the Transcriptome and Proteome.Sci Rep. 2017 Apr 4;7(1):642. doi: 10.1038/s41598-017-00516-x. Sci Rep. 2017. PMID: 28377578 Free PMC article.
-
Augmented oxygen-mediated transcriptional activation of cytochrome P450 (CYP)1A expression and increased susceptibilities to hyperoxic lung injury in transgenic mice carrying the human CYP1A1 or mouse 1A2 promoter in vivo.Biochem Biophys Res Commun. 2011 Apr 1;407(1):79-85. doi: 10.1016/j.bbrc.2011.02.113. Epub 2011 Mar 6. Biochem Biophys Res Commun. 2011. PMID: 21362406 Free PMC article.
-
Mice deficient in the gene for cytochrome P450 (CYP)1A1 are more susceptible than wild-type to hyperoxic lung injury: evidence for protective role of CYP1A1 against oxidative stress.Toxicol Sci. 2014 Sep;141(1):68-77. doi: 10.1093/toxsci/kfu106. Epub 2014 Jun 3. Toxicol Sci. 2014. PMID: 24893714 Free PMC article.
-
The role of cytochrome P450 (CYP) enzymes in hyperoxic lung injury.Expert Opin Drug Metab Toxicol. 2021 Feb;17(2):171-178. doi: 10.1080/17425255.2021.1853705. Epub 2020 Dec 13. Expert Opin Drug Metab Toxicol. 2021. PMID: 33215946 Free PMC article. Review.
-
Review of Ligand Specificity Factors for CYP1A Subfamily Enzymes from Molecular Modeling Studies Reported to-Date.Molecules. 2017 Jul 8;22(7):1143. doi: 10.3390/molecules22071143. Molecules. 2017. PMID: 28698457 Free PMC article. Review.
Cited by
-
Age- and sex-related changes in rat renal function and pathology following neonatal hyperoxia exposure.Physiol Rep. 2016 Aug;4(15):e12887. doi: 10.14814/phy2.12887. Physiol Rep. 2016. PMID: 27528005 Free PMC article.
-
Effect of neonatal hyperoxia followed by concentrated ambient ultrafine particle exposure on cumulative learning in C57Bl/6J mice.Neurotoxicology. 2018 Jul;67:234-244. doi: 10.1016/j.neuro.2018.06.006. Epub 2018 Jun 18. Neurotoxicology. 2018. PMID: 29920326 Free PMC article.
-
Molecular role of cytochrome P4501A enzymes inoxidative stress.Curr Opin Toxicol. 2020 Apr-Jun;20-21:77-84. doi: 10.1016/j.cotox.2020.07.001. Epub 2020 Jul 24. Curr Opin Toxicol. 2020. PMID: 33283080 Free PMC article.
-
Should we still use vitamin A to prevent bronchopulmonary dysplasia?J Perinatol. 2016 Aug;36(8):581-5. doi: 10.1038/jp.2016.76. Epub 2016 May 26. J Perinatol. 2016. PMID: 27228508 Review.
-
Former-preterm lambs have persistent alveolar simplification at 2 and 5 months corrected postnatal age.Am J Physiol Lung Cell Mol Physiol. 2018 Nov 1;315(5):L816-L833. doi: 10.1152/ajplung.00249.2018. Epub 2018 Sep 13. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 30211655 Free PMC article.
References
-
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi:10.1006/abio.1987.9999. - PubMed
-
- Couroucli XI, Liang Y-HW, Jiang W, Wang L, Barrios R, Yang P, Moorthy B. Prenatal administration of the cytochrome P4501A inducer, B-naphthoflavone (BNF), attenuates hyperoxic lung injury in newborn mice: implications for bronchopulmonary dysplasia (BPD) in premature infants. Toxicol. Appl. Pharmacol. 2011;256:83–94. doi:10.1016/j.taap.2011.06.018. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-HL-112516/HL/NHLBI NIH HHS/United States
- R01 HL088343/HL/NHLBI NIH HHS/United States
- R01 ES009132/ES/NIEHS NIH HHS/United States
- R01 ES019689/ES/NIEHS NIH HHS/United States
- U54 HD083092/HD/NICHD NIH HHS/United States
- R01-ES-019689/ES/NIEHS NIH HHS/United States
- R01 HL087174/HL/NHLBI NIH HHS/United States
- R01 ES-009132/ES/NIEHS NIH HHS/United States
- R01 HL112516/HL/NHLBI NIH HHS/United States
- P30 ES023512/ES/NIEHS NIH HHS/United States
- R01-HL-088343/HL/NHLBI NIH HHS/United States
- R01-HL-087174/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

