Activation of non-canonical TGF-β1 signaling indicates an autoimmune mechanism for bone marrow fibrosis in primary myelofibrosis
- PMID: 25703685
- PMCID: PMC4338409
- DOI: 10.1016/j.bcmd.2014.12.005
Activation of non-canonical TGF-β1 signaling indicates an autoimmune mechanism for bone marrow fibrosis in primary myelofibrosis
Abstract
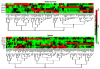
Primary myelofibrosis (PMF) is characterized by megakaryocyte hyperplasia, dysplasia and death with progressive reticulin/collagen fibrosis in marrow and hematopoiesis in extramedullary sites. The mechanism of fibrosis was investigated by comparing TGF-β1 signaling of marrow and spleen of patients with PMF and of non-diseased individuals. Expression of 39 (23 up-regulated and 16 down-regulated) and 38 (8 up-regulated and 30 down-regulated) TGF-β1 signaling genes was altered in the marrow and spleen of PMF patients, respectively. Abnormalities included genes of TGF-β1 signaling, cell cycling and abnormal in chronic myeloid leukemia (EVI1 and p21(CIP)) (both marrow and spleen) and Hedgehog (marrow only) and p53 (spleen only) signaling. Pathway analyses of these alterations predict an increased osteoblast differentiation, ineffective hematopoiesis and fibrosis driven by non-canonical TGF-β1 signaling in marrow and increased proliferation and defective DNA repair in spleen. Since activation of non-canonical TGF-β1 signaling is associated with fibrosis in autoimmune diseases, the hypothesis that fibrosis in PMF results from an autoimmune process triggered by dead megakaryocytes was tested by determining that PMF patients expressed plasma levels of mitochondrial DNA and anti-mitochondrial antibodies greater than normal controls. These data identify autoimmunity as a possible cause of marrow fibrosis in PMF.
Keywords: Autoimmunity; Inflammation; Myelofibrosis; TGF-β1.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
Fiorella Ciaffoni, Elena Cassella and Margherita Massa performed experiments and analyzed data.
Giovanni Barosi provided samples from PMF patients and normal donors and assured compliance of the study with institutional IRB.
Anna Rita Migliaccio, Lilian Varricchio and Giovanni Barosi designed research, analyzed the data and wrote the manuscript.
All the authors have read the manuscript, concur with its content and state that its content has not been submitted elsewhere.
Figures

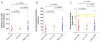

Similar articles
-
Characterization of the TGF-β1 signaling abnormalities in the Gata1low mouse model of myelofibrosis.Blood. 2013 Apr 25;121(17):3345-63. doi: 10.1182/blood-2012-06-439661. Epub 2013 Mar 5. Blood. 2013. PMID: 23462118 Free PMC article.
-
P-Selectin Sustains Extramedullary Hematopoiesis in the Gata1 low Model of Myelofibrosis.Stem Cells. 2016 Jan;34(1):67-82. doi: 10.1002/stem.2229. Epub 2015 Oct 23. Stem Cells. 2016. PMID: 26439305
-
A pathobiologic pathway linking thrombopoietin, GATA-1, and TGF-beta1 in the development of myelofibrosis.Blood. 2005 May 1;105(9):3493-501. doi: 10.1182/blood-2004-04-1320. Epub 2005 Jan 21. Blood. 2005. PMID: 15665119
-
Pathophysiological mechanisms operating in the development of myelofibrosis: role of megakaryocytes.Nouv Rev Fr Hematol (1978). 1982;24(4):221-6. Nouv Rev Fr Hematol (1978). 1982. PMID: 6292827 Review.
-
Pathogenesis of myelofibrosis: role of ineffective megakaryopoiesis and megakaryocyte components.Prog Clin Biol Res. 1984;154:427-54. Prog Clin Biol Res. 1984. PMID: 6089232 Review.
Cited by
-
Genetics and Pathogenetic Role of Inflammasomes in Philadelphia Negative Chronic Myeloproliferative Neoplasms: A Narrative Review.Int J Mol Sci. 2021 Jan 8;22(2):561. doi: 10.3390/ijms22020561. Int J Mol Sci. 2021. PMID: 33429941 Free PMC article. Review.
-
Role of Inflammatory Factors during Disease Pathogenesis and Stem Cell Transplantation in Myeloproliferative Neoplasms.Cancers (Basel). 2020 Aug 12;12(8):2250. doi: 10.3390/cancers12082250. Cancers (Basel). 2020. PMID: 32806517 Free PMC article. Review.
-
Inflammation as a Keystone of Bone Marrow Stroma Alterations in Primary Myelofibrosis.Mediators Inflamm. 2015;2015:415024. doi: 10.1155/2015/415024. Epub 2015 Nov 12. Mediators Inflamm. 2015. PMID: 26640324 Free PMC article. Review.
-
A novel interaction between megakaryocytes and activated fibrocytes increases TGF-β bioavailability in the Gata1(low) mouse model of myelofibrosis.Am J Blood Res. 2015 Dec 25;5(2):34-61. eCollection 2015. Am J Blood Res. 2015. PMID: 27069753 Free PMC article.
-
Monocytic Myeloid Derived Suppressor Cells in Hematological Malignancies.Int J Mol Sci. 2019 Nov 1;20(21):5459. doi: 10.3390/ijms20215459. Int J Mol Sci. 2019. PMID: 31683978 Free PMC article. Review.
References
-
- Ghosh AK, Quaggin SE, Vaughan DE. Molecular basis of organ fibrosis: potential therapeutic approaches. Exp Biol Med (Maywood) 2013;238:461–481. - PubMed
-
- Massague J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. - PubMed
-
- Fukasawa H, Yamamoto T, Togawa A, Ohashi N, Fujigaki Y, Oda T, Uchida C, Kitagawa K, Hattori T, Suzuki S, Kitagawa M, Hishida A. Down-regulation of Smad7 expression by ubiquitin-dependent degradation contributes to renal fibrosis in obstructive nephropathy in mice. Proc Natl Acad Sci U S A. 2004;101:8687–8692. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

