Influenza virus adaptation PB2-627K modulates nucleocapsid inhibition by the pathogen sensor RIG-I
- PMID: 25704008
- PMCID: PMC4359673
- DOI: 10.1016/j.chom.2015.01.005
Influenza virus adaptation PB2-627K modulates nucleocapsid inhibition by the pathogen sensor RIG-I
Abstract
The cytoplasmic RNA helicase RIG-I mediates innate sensing of RNA viruses. The genomes of influenza A virus (FLUAV) are encapsidated by the nucleoprotein and associated with RNA polymerase, posing potential barriers to RIG-I sensing. We show that RIG-I recognizes the 5'-triphosphorylated dsRNA on FLUAV nucleocapsids but that polymorphisms at position 627 of the viral polymerase subunit PB2 modulate RIG-I sensing. Compared to mammalian-adapted PB2-627K, avian FLUAV nucleocapsids possessing PB2-627E are prone to increased RIG-I recognition, and RIG-I-deficiency partially restores PB2-627E virus infection of mammalian cells. Heightened RIG-I sensing of PB2-627E nucleocapsids correlates with previously established lower affinity of 627E-containing PB2 for nucleoprotein and is increased by further nucleocapsid instability. The effect of RIG-I on PB2-627E nucleocapsids is independent of antiviral signaling, suggesting that RIG-I-nucleocapsid binding alone can inhibit infection. These results indicate that RIG-I is a direct avian FLUAV restriction factor and highlight nucleocapsid disruption as an antiviral strategy.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
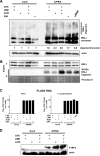


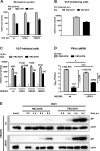

Comment in
-
RIG-I works double duty.Cell Host Microbe. 2015 Mar 11;17(3):285-287. doi: 10.1016/j.chom.2015.02.014. Cell Host Microbe. 2015. PMID: 25766287 Free PMC article.
Similar articles
-
Interactions between the influenza A virus RNA polymerase components and retinoic acid-inducible gene I.J Virol. 2014 Sep;88(18):10432-47. doi: 10.1128/JVI.01383-14. Epub 2014 Jun 18. J Virol. 2014. PMID: 24942585 Free PMC article.
-
Evidence for a novel mechanism of influenza A virus host adaptation modulated by PB2-627.FEBS J. 2019 Sep;286(17):3389-3400. doi: 10.1111/febs.14867. Epub 2019 Jul 5. FEBS J. 2019. PMID: 31034753
-
The effect of the PB2 mutation 627K on highly pathogenic H5N1 avian influenza virus is dependent on the virus lineage.J Virol. 2013 Sep;87(18):9983-96. doi: 10.1128/JVI.01399-13. Epub 2013 Jul 10. J Virol. 2013. PMID: 23843645 Free PMC article.
-
The catcher in the RIG-I.Cytokine. 2015 Nov;76(1):38-41. doi: 10.1016/j.cyto.2015.07.002. Epub 2015 Jul 10. Cytokine. 2015. PMID: 26168692 Review.
-
Standing on three legs: antiviral activities of RIG-I against influenza viruses.Curr Opin Immunol. 2016 Oct;42:71-75. doi: 10.1016/j.coi.2016.05.016. Epub 2016 Jun 16. Curr Opin Immunol. 2016. PMID: 27318973 Review.
Cited by
-
Antiviral RNAi in Insects and Mammals: Parallels and Differences.Viruses. 2019 May 16;11(5):448. doi: 10.3390/v11050448. Viruses. 2019. PMID: 31100912 Free PMC article. Review.
-
Exploring Potential Intermediates in the Cross-Species Transmission of Influenza A Virus to Humans.Viruses. 2024 Jul 14;16(7):1129. doi: 10.3390/v16071129. Viruses. 2024. PMID: 39066291 Free PMC article. Review.
-
Distinct and Orchestrated Functions of RNA Sensors in Innate Immunity.Immunity. 2020 Jul 14;53(1):26-42. doi: 10.1016/j.immuni.2020.03.017. Immunity. 2020. PMID: 32668226 Free PMC article. Review.
-
RIG-I triggers a signaling-abortive anti-SARS-CoV-2 defense in human lung cells.Nat Immunol. 2021 Jul;22(7):820-828. doi: 10.1038/s41590-021-00942-0. Epub 2021 May 11. Nat Immunol. 2021. PMID: 33976430
-
Influenza A Virus Panhandle Structure Is Directly Involved in RIG-I Activation and Interferon Induction.J Virol. 2015 Jun;89(11):6067-79. doi: 10.1128/JVI.00232-15. Epub 2015 Mar 25. J Virol. 2015. PMID: 25810557 Free PMC article.
References
-
- Cauldwell AV, Long JS, Moncorge O, Barclay WS. Viral determinants of influenza A virus host range. The Journal of general virology. 2014;95:1193–1210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

