Brilliant blue FCF is a nontoxic dye for saphenous vein graft marking that abrogates response to injury
- PMID: 25704409
- PMCID: PMC4544660
- DOI: 10.1016/j.jvs.2014.12.059
Brilliant blue FCF is a nontoxic dye for saphenous vein graft marking that abrogates response to injury
Abstract
Background: Injury to saphenous vein grafts during surgical preparation may contribute to the subsequent development of intimal hyperplasia, the primary cause of graft failure. Surgical skin markers currently used for vascular marking contain gentian violet and isopropanol, which damage tissue and impair physiologic functions. Brilliant blue FCF (FCF) is a nontoxic dye alternative that may also ameliorate preparation-induced injury.
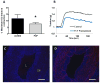
Methods: Porcine saphenous vein (PSV) was used to evaluate the effect of FCF on physiologic responses in a muscle bath. Cytotoxicity of FCF was measured using human umbilical venous smooth muscle cells. Effect of FCF on the development of intimal hyperplasia was evaluated in organ culture using PSV. Intracellular calcium fluxes and contractile responses were measured in response to agonists and inhibitors in rat aorta and human saphenous vein.
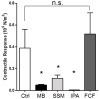
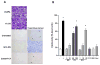
Results: Marking with FCF did not impair smooth muscle contractile responses and restored stretch injury-induced loss in smooth muscle contractility of PSV. Gentian violet has cytotoxic effects on human umbilical venous smooth muscle cells, whereas FCF is nontoxic. FCF inhibited intimal thickening in PSV in organ culture. Contraction induced by 2'(3')-O-(4-benzoylbenzoyl)adenosine 5'-triphosphate and intracellular calcium flux were inhibited by FCF, oxidized adenosine triphosphate, KN-62, and brilliant blue G, suggesting that FCF may inhibit the purinergic receptor P2X7.
Conclusions: Our studies indicated that FCF is a nontoxic marking dye for vein grafts that ameliorates vein graft injury and prevents intimal thickening, possibly due to P2X7 receptor inhibition. FCF represents a nontoxic alternative for vein graft marking and a potentially therapeutic approach to enhance outcome in autologous transplantation of human saphenous vein into the coronary and peripheral arterial circulation.
Copyright © 2016 Society for Vascular Surgery. All rights reserved.
Figures


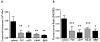

Similar articles
-
Brilliant blue FCF as an alternative dye for saphenous vein graft marking: effect on conduit function.JAMA Surg. 2014 Nov;149(11):1176-81. doi: 10.1001/jamasurg.2014.2029. JAMA Surg. 2014. PMID: 25251505 Free PMC article.
-
Use of Brilliant Blue FCF during vein graft preparation inhibits intimal hyperplasia.J Vasc Surg. 2016 Aug;64(2):471-478. doi: 10.1016/j.jvs.2015.02.028. J Vasc Surg. 2016. PMID: 27763268 Free PMC article.
-
P2X7R antagonism after subfailure overstretch injury of blood vessels reverses vasomotor dysfunction and prevents apoptosis.Purinergic Signal. 2017 Dec;13(4):579-590. doi: 10.1007/s11302-017-9585-0. Epub 2017 Sep 13. Purinergic Signal. 2017. PMID: 28905300 Free PMC article.
-
Role of the renin-angiotensin system in the pathogenesis of intimal hyperplasia: therapeutic potential for prevention of vein graft failure?Ann Vasc Surg. 2012 Nov;26(8):1130-44. doi: 10.1016/j.avsg.2011.12.001. Epub 2012 Mar 22. Ann Vasc Surg. 2012. PMID: 22445245 Free PMC article. Review.
-
Cell-permeant peptide inhibitors of vasospasm and intimal hyperplasia.Vascular. 2013 Feb;21(1):46-53. doi: 10.1258/vasc.2011.201203. Epub 2012 Oct 26. Vascular. 2013. PMID: 23104826 Free PMC article. Review.
Cited by
-
Adenosine triphosphate as a molecular mediator of the vascular response to injury.J Surg Res. 2017 Aug;216:80-86. doi: 10.1016/j.jss.2017.03.025. Epub 2017 Mar 31. J Surg Res. 2017. PMID: 28807217 Free PMC article.
-
Vein graft failure: from pathophysiology to clinical outcomes.Nat Rev Cardiol. 2016 Aug;13(8):451-70. doi: 10.1038/nrcardio.2016.76. Epub 2016 May 19. Nat Rev Cardiol. 2016. PMID: 27194091 Review.
-
Food Safety and Health Concerns of Synthetic Food Colors: An Update.Toxics. 2024 Jun 27;12(7):466. doi: 10.3390/toxics12070466. Toxics. 2024. PMID: 39058118 Free PMC article. Review.
-
Vascular surgical stretch injury leads to activation of P2X7 receptors and impaired endothelial function.PLoS One. 2017 Nov 14;12(11):e0188069. doi: 10.1371/journal.pone.0188069. eCollection 2017. PLoS One. 2017. PMID: 29136654 Free PMC article.
-
Traditional graft preparation decreases physiologic responses, diminishes viscoelasticity, and reduces cellular viability of the conduit: A porcine saphenous vein model.Vasc Med. 2016 Oct;21(5):413-421. doi: 10.1177/1358863X16649040. Epub 2016 May 23. Vasc Med. 2016. PMID: 27216870 Free PMC article.
References
-
- Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. Journal of vascular surgery. 2006;43:742–51. discussion 51. - PubMed
-
- Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson TB, Jr, Lorenz TJ, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA: the journal of the American Medical Association. 2005;294:2446–54. - PubMed
-
- Clowes AW, Reidy MA. Prevention of stenosis after vascular reconstruction: pharmacologic control of intimal hyperplasia--a review. J Vasc Surg. 1991;13:885–91. - PubMed
-
- LoGerfo FW, Quist WC, Cantelmo NL, Haudenschild CC. Integrity of vein grafts as a function of initial intimal and medial preservation. Circulation. 1983;68:II117–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

