Enteral nutrient deprivation in patients leads to a loss of intestinal epithelial barrier function
- PMID: 25704423
- PMCID: PMC4369405
- DOI: 10.1016/j.surg.2014.12.004
Enteral nutrient deprivation in patients leads to a loss of intestinal epithelial barrier function
Abstract
Objective: To investigate the effect of nutrient withdrawal on human intestinal epithelial barrier function (EBF). We hypothesized that unfed mucosa results in decreased EBF. This was tested in a series of surgical small intestinal resection specimens.
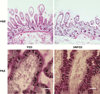
Design: Small bowel specifically excluding inflamed tissue, was obtained from pediatric patients (aged 2 days to 19 years) undergoing intestinal resection. EBF was assessed in Ussing chambers for transepithelial resistance (TER) and passage of fluorescein isothiocyanate (FITC)-dextran (4 kD). Tight junction and adherence junction proteins were imaged with immunofluorescence staining. Expression of Toll-like receptors (TLR) and inflammatory cytokines were measured in loop ileostomy takedowns in a second group of patients.
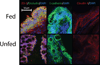
Results: Because TER increased with patient age (P < .01), results were stratified into infant versus teenage groups. Fed bowel had significantly greater TER versus unfed bowel (P < .05) in both age populations. Loss of EBF was also observed by an increase in FITC-dextran permeation in enteral nutrient-deprived segments (P < .05). Immunofluorescence staining showed marked declines in intensity of ZO-1, occludin, E-cadherin, and claudin-4 in unfed intestinal segments, as well as a loss of structural formation of tight junctions. Analysis of cytokine and TLR expression showed significant increases in tumor necrosis factor (TNF)-α and TLR4 in unfed segments of bowel compared with fed segments from the same individual.
Conclusion: EBF declined in unfed segments of human small bowel. This work represents the first direct examination of EBF from small bowel derived from nutrient-deprived humans and may explain the increased incidence of infectious complications seen in patients not receiving enteral feeds.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

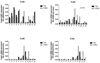
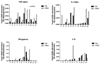
References
-
- Braga M, Ljungqvist O, Soeters P, Fearon K, Weimann A, et al. ESPEN Guidelines on Parenteral Nutrition: surgery. Clin Nutr. 2009;28:378–386. - PubMed
-
- Duro D, Kamin D, Duggan C. Overview of pediatric short bowel syndrome. J Pediatr Gastroenterol Nutr. 2008;47(Suppl 1):S33–S36. - PubMed
-
- (AHRQ) AfHRaQ, editor. 2010. HCUPnet National Center for Health Statistics Data on PN and EN Use.
-
- Torres C, Sudan D, Vanderhoof J, Grant W, Botha J, et al. Role of an intestinal rehabilitation program in the treatment of advanced intestinal failure. J Pediatr Gastroenterol Nutr. 2007;45:204–212. - PubMed
-
- Cowles RA, Ventura KA, Martinez M, Lobritto SJ, Harren PA, et al. Reversal of intestinal failure-associated liver disease in infants and children on parenteral nutrition: experience with 93 patients at a referral center for intestinal rehabilitation. J Pediatr Surg. 2010;45:84–87. discussion 87–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

