Analysis of p53 transactivation domain mutants reveals Acad11 as a metabolic target important for p53 pro-survival function
- PMID: 25704813
- PMCID: PMC4365998
- DOI: 10.1016/j.celrep.2015.01.043
Analysis of p53 transactivation domain mutants reveals Acad11 as a metabolic target important for p53 pro-survival function
Abstract
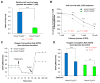
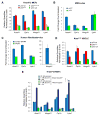
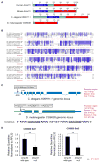
The p53 tumor suppressor plays a key role in maintaining cellular integrity. In response to diverse stress signals, p53 can trigger apoptosis to eliminate damaged cells or cell-cycle arrest to enable cells to cope with stress and survive. However, the transcriptional networks underlying p53 pro-survival function are incompletely understood. Here, we show that in oncogenic-Ras-expressing cells, p53 promotes oxidative phosphorylation (OXPHOS) and cell survival upon glucose starvation. Analysis of p53 transcriptional activation domain mutants reveals that these responses depend on p53 transactivation function. Using gene expression profiling and ChIP-seq analysis, we identify several p53-inducible fatty acid metabolism-related genes. One such gene, Acad11, encoding a protein involved in fatty acid oxidation, is required for efficient OXPHOS and cell survival upon glucose starvation. This study provides new mechanistic insight into the pro-survival function of p53 and suggests that targeting this pathway may provide a strategy for therapeutic intervention based on metabolic perturbation.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Assaily W, Rubinger DA, Wheaton K, Lin Y, Ma W, Xuan W, Brown-Endres L, Tsuchihara K, Mak TW, Benchimol S. ROS-mediated p53 induction of Lpin1 regulates fatty acid oxidation in response to nutritional stress. Mol Cell. 2011;44:491–501. - PubMed
-
- Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, Gottlieb E, Vousden KH. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

