Vascular endothelial growth factor receptor 3 controls neural stem cell activation in mice and humans
- PMID: 25704818
- PMCID: PMC4685253
- DOI: 10.1016/j.celrep.2015.01.049
Vascular endothelial growth factor receptor 3 controls neural stem cell activation in mice and humans
Abstract
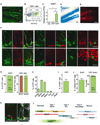
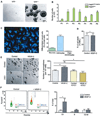
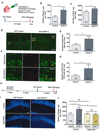
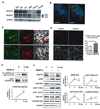
Neural stem cells (NSCs) continuously produce new neurons within the adult mammalian hippocampus. NSCs are typically quiescent but activated to self-renew or differentiate into neural progenitor cells. The molecular mechanisms of NSC activation remain poorly understood. Here, we show that adult hippocampal NSCs express vascular endothelial growth factor receptor (VEGFR) 3 and its ligand VEGF-C, which activates quiescent NSCs to enter the cell cycle and generate progenitor cells. Hippocampal NSC activation and neurogenesis are impaired by conditional deletion of Vegfr3 in NSCs. Functionally, this is associated with compromised NSC activation in response to VEGF-C and physical activity. In NSCs derived from human embryonic stem cells (hESCs), VEGF-C/VEGFR3 mediates intracellular activation of AKT and ERK pathways that control cell fate and proliferation. These findings identify VEGF-C/VEGFR3 signaling as a specific regulator of NSC activation and neurogenesis in mammals.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


Comment in
-
Cell autonomous Vegf-C/Vegfr3 signaling in adult neural stem cells.Oncotarget. 2015 Nov 24;6(37):39387-8. doi: 10.18632/oncotarget.6325. Oncotarget. 2015. PMID: 26575019 Free PMC article. No abstract available.
Similar articles
-
Molecular controls of lymphatic VEGFR3 signaling.Arterioscler Thromb Vasc Biol. 2015 Feb;35(2):421-9. doi: 10.1161/ATVBAHA.114.304881. Epub 2014 Dec 18. Arterioscler Thromb Vasc Biol. 2015. PMID: 25524775 Free PMC article.
-
Vegfr3 activation of Pkd2l1+ CSF-cNs triggers the neural stem cell response in spinal cord injury.Cell Signal. 2025 Jun;130:111675. doi: 10.1016/j.cellsig.2025.111675. Epub 2025 Feb 20. Cell Signal. 2025. PMID: 39986360
-
Protein S Regulates Neural Stem Cell Quiescence and Neurogenesis.Stem Cells. 2017 Mar;35(3):679-693. doi: 10.1002/stem.2522. Epub 2016 Nov 8. Stem Cells. 2017. PMID: 27753164
-
Neural stem cell survival factors.Arch Biochem Biophys. 2013 Jun;534(1-2):71-87. doi: 10.1016/j.abb.2013.02.004. Epub 2013 Mar 5. Arch Biochem Biophys. 2013. PMID: 23470250 Review.
-
Maintenance and differentiation of neural stem cells.Wiley Interdiscip Rev Syst Biol Med. 2011 Jan-Feb;3(1):107-14. doi: 10.1002/wsbm.100. Wiley Interdiscip Rev Syst Biol Med. 2011. PMID: 21061307 Review.
Cited by
-
Waking up quiescent neural stem cells: Molecular mechanisms and implications in neurodevelopmental disorders.PLoS Genet. 2020 Apr 23;16(4):e1008653. doi: 10.1371/journal.pgen.1008653. eCollection 2020 Apr. PLoS Genet. 2020. PMID: 32324743 Free PMC article. Review.
-
Cell autonomous Vegf-C/Vegfr3 signaling in adult neural stem cells.Oncotarget. 2015 Nov 24;6(37):39387-8. doi: 10.18632/oncotarget.6325. Oncotarget. 2015. PMID: 26575019 Free PMC article. No abstract available.
-
Molecular heterogeneity of glioblastomas: does location matter?Oncotarget. 2016 Jan 5;7(1):902-13. doi: 10.18632/oncotarget.6433. Oncotarget. 2016. PMID: 26637806 Free PMC article.
-
Mapping the cellular expression patterns of vascular endothelial growth factor aa and bb genes and their receptors in the adult zebrafish brain during constitutive and regenerative neurogenesis.Neural Dev. 2024 Sep 12;19(1):17. doi: 10.1186/s13064-024-00195-1. Neural Dev. 2024. PMID: 39267104 Free PMC article.
-
EGFL7 loss correlates with increased VEGF-D expression, upregulating hippocampal adult neurogenesis and improving spatial learning and memory.Cell Mol Life Sci. 2023 Jan 30;80(2):54. doi: 10.1007/s00018-023-04685-z. Cell Mol Life Sci. 2023. PMID: 36715759 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

