Enantioselective Synthesis of Spiro[cyclohexane-1,3'-indolin]-2'-ones Containing Multiple Stereocenters via Organocatalytic Michael/Aldol Cascade Reactions
- PMID: 25705059
- PMCID: PMC4335313
- DOI: 10.1016/j.tetlet.2013.02.030
Enantioselective Synthesis of Spiro[cyclohexane-1,3'-indolin]-2'-ones Containing Multiple Stereocenters via Organocatalytic Michael/Aldol Cascade Reactions
Abstract
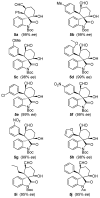
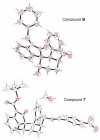
Organocatalytic reactions of 3-olefinic oxindoles and pentane-1,5-dial were investigated to provide access to substituted spirocyclohexane oxindoles via Michael/Aldol cascade reactions. Of particular interest, we have examined the stereochemical outcome of electron withdrawing and electron-donating groups on the oxindole ring nitrogen. Interestingly, we have observed that the N-protecting group on the oxindole has critical effect on aldol ring closure leading to ultimate stereochemical outcome of the hydroxyl center. The overall process is quite efficient and afforded products with multiple stereocenters in high yields and excellent enantioselectivities (>99% ee).
Keywords: Aldol reaction; Asymmetric catalysis; Michael reaction; Organocatalyst; Spiroindole.
Figures
Similar articles
-
Organocatalytic Enantioselective Michael-Michael-Michael-Aldol Condensation Reactions: Control of Six Stereocenters in a Quadruple-Cascade Asymmetric Synthesis of Polysubstituted Spirocyclic Oxindoles.Org Lett. 2017 Nov 17;19(22):6112-6115. doi: 10.1021/acs.orglett.7b02962. Epub 2017 Nov 1. Org Lett. 2017. PMID: 29090937
-
Enantioselective Construction of Spirocyclic Oxindole Derivatives with Multiple Stereocenters via an Organocatalytic Michael/Aldol/Hemiacetalization Cascade Reaction.Org Lett. 2016 May 20;18(10):2387-90. doi: 10.1021/acs.orglett.6b00873. Epub 2016 May 4. Org Lett. 2016. PMID: 27145022
-
Asymmetric Michael addition reaction of 3-substituted-N-Boc oxindoles to activated terminal alkenes catalyzed by a bifunctional tertiary-amine thiourea catalyst.Org Biomol Chem. 2010 Jan 7;8(1):77-82. doi: 10.1039/b918644a. Epub 2009 Oct 29. Org Biomol Chem. 2010. PMID: 20024135
-
[Development of boomerang-type intramolecular cascade reactions and application to natural product synthesis].Yakugaku Zasshi. 2001 Dec;121(12):887-98. doi: 10.1248/yakushi.121.887. Yakugaku Zasshi. 2001. PMID: 11766403 Review. Japanese.
-
Progress in Catalytic Asymmetric Reactions with 7-Azaindoline as the Directing Group.Molecules. 2023 Dec 1;28(23):7898. doi: 10.3390/molecules28237898. Molecules. 2023. PMID: 38067627 Free PMC article. Review.
Cited by
-
Molecular diversity of spirooxindoles. Synthesis and biological activity.Mol Divers. 2016 Feb;20(1):299-344. doi: 10.1007/s11030-015-9629-8. Epub 2015 Sep 29. Mol Divers. 2016. PMID: 26419598 Review.
-
Organocatalyzed [4 + 2] cycloaddition of α,β-unsaturated ketones and isatylidene malononitrile: accessing spiro[3-arylcyclohexanone]oxindole derivatives.RSC Adv. 2024 Jan 17;14(5):2873-2877. doi: 10.1039/d3ra07652k. eCollection 2024 Jan 17. RSC Adv. 2024. PMID: 38239455 Free PMC article.
-
HOAc-Mediated Domino Diels-Alder Reaction for Synthesis of Spiro[cyclohexane-1,3'-indolines] in Ionic Liquid [Bmim]Br.ACS Omega. 2018 May 21;3(5):5406-5416. doi: 10.1021/acsomega.8b00464. eCollection 2018 May 31. ACS Omega. 2018. PMID: 31458748 Free PMC article.
References
-
-
For selected examples, see: Serradeil-Le Gal C, Lacour C, Valette G, Garcia G, Foulon L, Galindo G, Bankir L, Pouzet B, Guillon G, Barberis C, Chicot D, Jard S, Vilain P, Garcia C, Marty E, Raufaste D, Brossard G, Nisato D, Maffrand JP, Le Fur G. J. Clin. Invest. 1996;98:2729. Venkatesan H, Davis MC, Altas Y, Snyder JP, Liotta DC. J. Org. Chem. 2001;66:3653.
-
-
- Beccalli EM, Clerici F, Gelmi ML. Tetrahedron. 2003;59:4615.
- Liu J-J, Zhang Z. PCT Int. Appl. WO2008/055812. Hoffmann-LaRoche AG; 2008. “Spiroindolinone derivatives”.
-
-
For selected reviews, see: Dounay AB, Overman LE. Chem. Rev. 2003;103:2945. For selected publications, see: Madin A, ODonnell CJ, Oh T, Old DW, Overman LE, Sharp MJ. J. Am. Chem. Soc. 2005;127:18054.
-
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources