Enantioselective Synthesis of Spiro[cyclohexane-1,3'-indolin]-2'-ones Containing Multiple Stereocenters via Organocatalytic Michael/Aldol Cascade Reactions
- PMID: 25705059
- PMCID: PMC4335313
- DOI: 10.1016/j.tetlet.2013.02.030
Enantioselective Synthesis of Spiro[cyclohexane-1,3'-indolin]-2'-ones Containing Multiple Stereocenters via Organocatalytic Michael/Aldol Cascade Reactions
Abstract
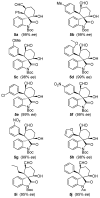
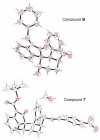
Organocatalytic reactions of 3-olefinic oxindoles and pentane-1,5-dial were investigated to provide access to substituted spirocyclohexane oxindoles via Michael/Aldol cascade reactions. Of particular interest, we have examined the stereochemical outcome of electron withdrawing and electron-donating groups on the oxindole ring nitrogen. Interestingly, we have observed that the N-protecting group on the oxindole has critical effect on aldol ring closure leading to ultimate stereochemical outcome of the hydroxyl center. The overall process is quite efficient and afforded products with multiple stereocenters in high yields and excellent enantioselectivities (>99% ee).
Keywords: Aldol reaction; Asymmetric catalysis; Michael reaction; Organocatalyst; Spiroindole.
Figures
References
-
-
For selected examples, see: Serradeil-Le Gal C, Lacour C, Valette G, Garcia G, Foulon L, Galindo G, Bankir L, Pouzet B, Guillon G, Barberis C, Chicot D, Jard S, Vilain P, Garcia C, Marty E, Raufaste D, Brossard G, Nisato D, Maffrand JP, Le Fur G. J. Clin. Invest. 1996;98:2729. Venkatesan H, Davis MC, Altas Y, Snyder JP, Liotta DC. J. Org. Chem. 2001;66:3653.
-
-
- Beccalli EM, Clerici F, Gelmi ML. Tetrahedron. 2003;59:4615.
- Liu J-J, Zhang Z. PCT Int. Appl. WO2008/055812. Hoffmann-LaRoche AG; 2008. “Spiroindolinone derivatives”.
-
-
For selected reviews, see: Dounay AB, Overman LE. Chem. Rev. 2003;103:2945. For selected publications, see: Madin A, ODonnell CJ, Oh T, Old DW, Overman LE, Sharp MJ. J. Am. Chem. Soc. 2005;127:18054.
-
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources