Understanding dynamic changes in live cell adhesion with neutron reflectometry
- PMID: 25705067
- PMCID: PMC4334466
- DOI: 10.1142/S0217984914300154
Understanding dynamic changes in live cell adhesion with neutron reflectometry
Abstract
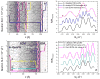
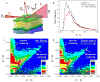
Neutron reflectometry (NR) was used to examine various live cells adhesion to quartz substrates under different environmental conditions, including flow stress. To the best of our knowledge, these measurements represent the first successful visualization and quantization of the interface between live cells and a substrate with sub-nanometer resolution. In our first experiments, we examined live mouse fibroblast cells as opposed to past experiments using supported lipids, proteins, or peptide layers with no associated cells. We continued the NR studies of cell adhesion by investigating endothelial monolayers and glioblastoma cells under dynamic flow conditions. We demonstrated that neutron reflectometry is a powerful tool to study the strength of cellular layer adhesion in living tissues, which is a key factor in understanding the physiology of cell interactions and conditions leading to abnormal or disease circumstances. Continuative measurements, such as investigating changes in tumor cell - surface contact of various glioblastomas, could impact advancements in tumor treatments. In principle, this can help us to identify changes that correlate with tumor invasiveness. Pursuit of these studies can have significant medical impact on the understanding of complex biological problems and their effective treatment, e.g. for the development of targeted anti-invasive therapies.
Keywords: adhesion; cells; endothelial monolayer; glioblastomas; neutron scattering; shear stress.
Figures
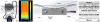
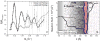




Similar articles
-
Mouse fibroblast cell adhesion studied by neutron reflectometry.Biophys J. 2010 Mar 3;98(5):793-9. doi: 10.1016/j.bpj.2009.11.019. Biophys J. 2010. PMID: 20197032 Free PMC article.
-
Tuning endothelial monolayer adhesion: a neutron reflectivity study.Am J Physiol Lung Cell Mol Physiol. 2014 Jan 1;306(1):L1-9. doi: 10.1152/ajplung.00160.2013. Epub 2013 Oct 25. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 24163142 Free PMC article.
-
Macromolecular crowding: chemistry and physics meet biology (Ascona, Switzerland, 10-14 June 2012).Phys Biol. 2013 Aug;10(4):040301. doi: 10.1088/1478-3975/10/4/040301. Epub 2013 Aug 2. Phys Biol. 2013. PMID: 23912807
-
How neutron scattering techniques benefit investigating structures and dynamics of monoclonal antibody.Biochim Biophys Acta Gen Subj. 2022 Nov;1866(11):130206. doi: 10.1016/j.bbagen.2022.130206. Epub 2022 Jul 21. Biochim Biophys Acta Gen Subj. 2022. PMID: 35872327 Review.
-
Structural Investigations of Protein-Lipid Complexes Using Neutron Scattering.Methods Mol Biol. 2019;2003:201-251. doi: 10.1007/978-1-4939-9512-7_11. Methods Mol Biol. 2019. PMID: 31218621 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
