Adipose stem cell microbeads as production sources for chondrogenic growth factors
- PMID: 25705097
- PMCID: PMC4329461
- DOI: 10.46582/jsrm.1002007
Adipose stem cell microbeads as production sources for chondrogenic growth factors
Abstract
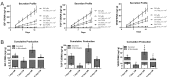
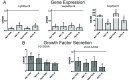
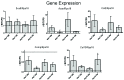
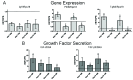
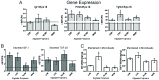
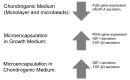
Microencapsulating stem cells in injectable microbeads can enhance delivery and localization, but their ability to act as growth factor production sources is still unknown. To address this concern, growth factor mRNA levels and production from alginate microbeads with encapsulated human adipose stem cells (ASC microbeads) cultured in both growth and chondrogenic media (GM and CM) were measured over a two week period. Human ASCs in microbeads were either commercially purchased (Lonza) or isolated from six human donors and compared to human ASCs on tissue culture polystyrene (TCPS). The effects of crosslinking and alginate compositions on growth factor mRNA levels and production were also determined. Secretion profiles of IGF-I, TGF-β3 and VEGF-A from commercial human ASC microbeads were linear and at a significantly higher rate than TCPS cultures over two weeks. For human ASCs derived from different donors, microencapsulation increased pthlh and both IGF-I and TGF-β3 secretion. CM decreased fgf2 and VEGF-A secretion from ASC microbeads derived from the same donor population. Crosslinking microbeads in BaCl2 instead of CaCl2 did not eliminate microencapsulation's beneficial effects, but did decrease IGF-I production. Increasing the guluronate content of the alginate microbead increased IGF-I retention. Decreasing alginate molecular weight eliminated the effects microencapsulation had on increasing IGF-I secretion. This study demonstrated that microencapsulation can enhance chondrogenic growth factor production and that chondrogenic medium treatment can decrease angiogenic growth factor production from ASCs, making these cells a potential source for paracrine factors that can stimulate cartilage regeneration.
Keywords: Adipose stem cells; Alginate; Cartilage; Growth factor delivery; Microencapsulation.
Figures

Similar articles
-
Tailoring adipose stem cell trophic factor production with differentiation medium components to regenerate chondral defects.Tissue Eng Part A. 2013 Jun;19(11-12):1451-64. doi: 10.1089/ten.TEA.2012.0233. Epub 2013 Mar 28. Tissue Eng Part A. 2013. PMID: 23350662 Free PMC article.
-
Adipose stem cells can secrete angiogenic factors that inhibit hyaline cartilage regeneration.Stem Cell Res Ther. 2012 Aug 24;3(4):35. doi: 10.1186/scrt126. Stem Cell Res Ther. 2012. PMID: 22920724 Free PMC article.
-
Impact of collagen-alginate composition from microbead morphological properties to microencapsulated canine adipose tissue-derived mesenchymal stem cell activities.J Biomater Sci Polym Ed. 2018 May-Jun;29(7-9):1042-1052. doi: 10.1080/09205063.2017.1399002. Epub 2017 Nov 6. J Biomater Sci Polym Ed. 2018. PMID: 29082833
-
Development of a cell delivery system using alginate microbeads for tissue regeneration.J Mater Chem B. 2016 May 28;4(20):3515-3525. doi: 10.1039/c6tb00035e. Epub 2016 Mar 21. J Mater Chem B. 2016. PMID: 32263385
-
Controlled release of rat adipose-derived stem cells from alginate microbeads.Biomaterials. 2013 Nov;34(33):8172-84. doi: 10.1016/j.biomaterials.2013.07.017. Epub 2013 Jul 29. Biomaterials. 2013. PMID: 23906513
Cited by
-
Injectable alginate-microencapsulated canine adipose tissue-derived mesenchymal stem cells for enhanced viable cell retention.J Vet Med Sci. 2017 Mar 18;79(3):492-501. doi: 10.1292/jvms.16-0456. Epub 2017 Jan 6. J Vet Med Sci. 2017. PMID: 28070061 Free PMC article.
-
Potential and recent advances of microcarriers in repairing cartilage defects.J Orthop Translat. 2021 Jan 13;27:101-109. doi: 10.1016/j.jot.2020.10.005. eCollection 2021 Mar. J Orthop Translat. 2021. PMID: 33520655 Free PMC article. Review.
-
Predominant control of PDGF/PDGF receptor signaling in the migration and proliferation of human adipose‑derived stem cells under culture conditions with a combination of growth factors.Exp Ther Med. 2024 Feb 21;27(4):156. doi: 10.3892/etm.2024.12444. eCollection 2024 Apr. Exp Ther Med. 2024. PMID: 38476902 Free PMC article.
-
3D Culture of MSCs on a Gelatin Microsphere in a Dynamic Culture System Enhances Chondrogenesis.Int J Mol Sci. 2020 Apr 13;21(8):2688. doi: 10.3390/ijms21082688. Int J Mol Sci. 2020. PMID: 32294921 Free PMC article.
-
Microcarriers in application for cartilage tissue engineering: Recent progress and challenges.Bioact Mater. 2022 Jan 25;17:81-108. doi: 10.1016/j.bioactmat.2022.01.033. eCollection 2022 Nov. Bioact Mater. 2022. PMID: 35386447 Free PMC article. Review.
References
-
- Gimble J, Guilak F.Adipose-derived adult stem cells: isolation, characterization and differentiation potential. Cytotherapy 2003; 5(5):362-9. - PubMed
-
- Moyer HR, Kinney RC, Singh KA, Williams JK, Schwartz Z, Boyan BD.Alginate microencapsulation technology for the percutaneous delivery of adipose-derived stem cells. Ann Plast Surg 2010; 65(5):497-503. - PubMed
-
- Takahashi T, Ogasawara T, Kishimoto J, Liu G, Asato H, Nakatsuka T, Uchinuma E, Nakamura K, Kawaguchi H, Chung UI, Takato T, Hoshi K.Synergistic effects of FGF-2 with insulin or IGF-I on the proliferation of human auricular chondrocytes. Cell Transplant 2005; 14(9):683-93. - PubMed
-
- Liu Z, Lavine KJ, Hung IH, Ornitz DM.FGF18 is required for early chondrocyte proliferation, hypertrophy and vascular invasion of the growth plate. Dev Biol 2007; 302(1):80-91. - PubMed
-
- Hanada K, Solchaga LA, Caplan AI, Hering TM, Goldberg VM, Yoo JU, Johnstone B.BMP-2 induction and TGF-beta 1 modulation of rat periosteal cell chondrogenesis. J Cell Biochem 2001; 81 (2):284-94. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
