Millimeter Wave Treatment Inhibits Apoptosis of Chondrocytes via Regulation Dynamic Equilibrium of Intracellular Free Ca (2+)
- PMID: 25705239
- PMCID: PMC4325209
- DOI: 10.1155/2015/464161
Millimeter Wave Treatment Inhibits Apoptosis of Chondrocytes via Regulation Dynamic Equilibrium of Intracellular Free Ca (2+)
Abstract
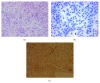
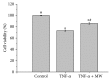
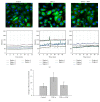
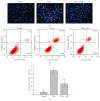
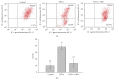
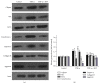
The molecular mechanisms of TNF-α-induced apoptosis of chondrocyte and the role of Ca(2+) mediating the effects of MW on TNF-α-induced apoptosis of chondrocytes remained unclear. In this study, we investigated the molecular mechanism underlying inhibiting TNF-α-induced chondrocytes apoptosis of MW. MTT assay, DAPI, and flow cytometry demonstrated that MW significantly increased cell activity and inhibited chromatin condensation accompanying the loss of plasma membrane asymmetry and the collapse of mitochondrial membrane potential. Our results also indicated that MW reduced the elevation of [Ca(2+)] i in chondrocytes by LSCM. Moreover, MW suppressed the protein levels of calpain, Bax, cytochrome c, and caspase-3, while the expressions of Bcl-2, collagen II, and aggrecan were increased. Our evidences indicated that MW treatment inhibited the apoptosis of chondrocytes through depression of [Ca(2+)] i . It also inhibited calpain activation, which mediated Bax cleavage and cytochrome c release and initiated the apoptotic execution phase. In addition, MW treatment increased the expression of collagen II and aggrecan of chondrocytes.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

