H2A.Z: a molecular rheostat for transcriptional control
- PMID: 25705384
- PMCID: PMC4311278
- DOI: 10.12703/P7-01
H2A.Z: a molecular rheostat for transcriptional control
Abstract
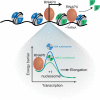
The replacement of nucleosomal H2A with the histone variant H2A.Z is critical for regulating DNA-mediated processes across eukaryotes and for early development of multicellular organisms. How this variant performs these seemingly diverse roles has remained largely enigmatic. Here, we discuss recent mechanistic insights that have begun to reveal how H2A.Z functions as a molecular rheostat for gene control. We focus on specific examples in metazoans as a model for understanding how H2A.Z integrates information from histone post-translational modifications, other histone variants, and transcription factors (TFs) to regulate proper induction of gene expression programs in response to cellular cues. Finally, we propose a general model of how H2A.Z incorporation regulates chromatin states in diverse processes.
Figures


Similar articles
-
Post-Translational Modifications of H2A Histone Variants and Their Role in Cancer.Cancers (Basel). 2018 Feb 27;10(3):59. doi: 10.3390/cancers10030059. Cancers (Basel). 2018. PMID: 29495465 Free PMC article. Review.
-
Transcriptional and epigenetic functions of histone variant H2A.Z.Biochem Cell Biol. 2009 Feb;87(1):19-25. doi: 10.1139/O08-117. Biochem Cell Biol. 2009. PMID: 19234520 Review.
-
H2A.Z-Mediated Genome-Wide Chromatin Specialization.Curr Genomics. 2007 Mar;8(1):59-66. doi: 10.2174/138920207780076965. Curr Genomics. 2007. PMID: 18645626 Free PMC article.
-
Histone H2A/H2B chaperones: from molecules to chromatin-based functions in plant growth and development.Plant J. 2015 Jul;83(1):78-95. doi: 10.1111/tpj.12830. Epub 2015 Apr 8. Plant J. 2015. PMID: 25781491 Review.
-
SUMO modification system facilitates the exchange of histone variant H2A.Z-2 at DNA damage sites.Nucleus. 2018 Jan 1;9(1):87-94. doi: 10.1080/19491034.2017.1395543. Epub 2017 Dec 14. Nucleus. 2018. PMID: 29095668 Free PMC article.
Cited by
-
Nucleosome mobility and the regulation of gene expression: Insights from single-molecule studies.Protein Sci. 2017 Jul;26(7):1266-1277. doi: 10.1002/pro.3159. Epub 2017 Apr 2. Protein Sci. 2017. PMID: 28329910 Free PMC article. Review.
-
KLU suppresses megasporocyte cell fate through SWR1-mediated activation of WRKY28 expression in Arabidopsis.Proc Natl Acad Sci U S A. 2018 Jan 16;115(3):E526-E535. doi: 10.1073/pnas.1716054115. Epub 2017 Dec 29. Proc Natl Acad Sci U S A. 2018. PMID: 29288215 Free PMC article.
-
A dual role for H2A.Z.1 in modulating the dynamics of RNA polymerase II initiation and elongation.Nat Struct Mol Biol. 2021 May;28(5):435-442. doi: 10.1038/s41594-021-00589-3. Epub 2021 May 10. Nat Struct Mol Biol. 2021. PMID: 33972784
-
Competitive Chemical Reaction Kinetic Model of Nucleosome Assembly Using the Histone Variant H2A.Z and H2A In Vitro.Int J Mol Sci. 2023 Oct 31;24(21):15846. doi: 10.3390/ijms242115846. Int J Mol Sci. 2023. PMID: 37958827 Free PMC article.
-
Neural progenitor cells mediated by H2A.Z.2 regulate microglial development via Cxcl14 in the embryonic brain.Proc Natl Acad Sci U S A. 2019 Nov 26;116(48):24122-24132. doi: 10.1073/pnas.1913978116. Epub 2019 Nov 11. Proc Natl Acad Sci U S A. 2019. PMID: 31712428 Free PMC article.

