Crystal structure of γ-ethyl-l-glutamate N-carb-oxy anhydride
- PMID: 25705466
- PMCID: PMC4331880
- DOI: 10.1107/S2056989014027170
Crystal structure of γ-ethyl-l-glutamate N-carb-oxy anhydride
Abstract
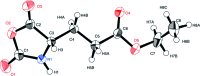
In the title compound (alternative name N-carboxy-l-glutamic anhydride γ-ethyl ester), C8H11NO5, the oxazolidine ring is essentially planar, with a maximum deviation of 0.019 (2) Å. In the crystal, mol-ecules are linked by N-H⋯O hydrogen bonds between the imino group and the carbonyl O atom in the ethyl ester group, forming a tape structure along the c-axis direction. The oxazolidine rings of adjacent tapes are arranged into a layer parallel to the ac plane. This arrangement is favourable for the polymerization of the title compound in the solid state.
Keywords: amino acid N-carboxy anhydrides; crystal structure; hydrogen bonding; solid-state polymerization.
Figures


Similar articles
-
Crystal structure of γ-methyl l-glutamate N-carb-oxy anhydride.Acta Crystallogr E Crystallogr Commun. 2015 Jan 1;71(Pt 1):48-50. doi: 10.1107/S2056989014026917. eCollection 2015 Jan 1. Acta Crystallogr E Crystallogr Commun. 2015. PMID: 25705448 Free PMC article.
-
Crystal structure of O-benzyl-l-tyrosine N-carb-oxy anhydride.Acta Crystallogr E Crystallogr Commun. 2017 Mar 21;73(Pt 4):553-555. doi: 10.1107/S2056989017004236. eCollection 2017 Apr 1. Acta Crystallogr E Crystallogr Commun. 2017. PMID: 28435719 Free PMC article.
-
Crystal structure of β-benzyl dl-aspartate N-carb-oxyanhydride.Acta Crystallogr E Crystallogr Commun. 2017 Feb 24;73(Pt 3):445-447. doi: 10.1107/S2056989017003024. eCollection 2017 Mar 1. Acta Crystallogr E Crystallogr Commun. 2017. PMID: 28316828 Free PMC article.
-
Crystal structure of 4-oxo-4H-chromene-3-carb-oxy-lic acid.Acta Crystallogr E Crystallogr Commun. 2015 Jul 17;71(Pt 8):o580-1. doi: 10.1107/S2056989015013456. eCollection 2015 Aug 1. Acta Crystallogr E Crystallogr Commun. 2015. PMID: 26396807 Free PMC article.
-
Crystal structure of (2S,4R)-ethyl 4-nitro-methyl-1-[(S)-1-phenyl-eth-yl]-6-sulfanyl-idene-piperidine-2-carboxyl-ate.Acta Crystallogr E Crystallogr Commun. 2015 Jan 1;71(Pt 1):o41-2. doi: 10.1107/S2056989014026711. eCollection 2015 Jan 1. Acta Crystallogr E Crystallogr Commun. 2015. PMID: 25705497 Free PMC article.
References
-
- Burla, M. C., Caliandro, R., Camalli, M., Carrozzini, B., Cascarano, G. L., De Caro, L., Giacovazzo, C., Polidori, G. & Spagna, R. (2005). J. Appl. Cryst. 38, 381–388.
-
- Groom, C. R. & Allen, F. H. (2014). Angew. Chem. Int. Ed. 53, 662–671. - PubMed
-
- Kanazawa, H. (1992). Polymer, 33, 2557–2566.
-
- Kanazawa, H. (1998). Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A. Mol. Cryst. Liq. Cryst. 313, 205–210.
-
- Kanazawa, H., Inada, A. & Kawana, N. (2006). Macromol. Symp. 242, 104–112.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
