Retroviral Integrase Structure and DNA Recombination Mechanism
- PMID: 25705574
- PMCID: PMC4334468
- DOI: 10.1128/microbiolspec.MDNA3-0024-2014.
Retroviral Integrase Structure and DNA Recombination Mechanism
Abstract
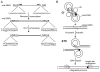
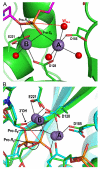
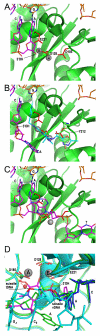
Due to the importance of human immunodeficiency virus type 1 (HIV-1) integrase as a drug target, the biochemistry and structural aspects of retroviral DNA integration have been the focus of intensive research during the past three decades. The retroviral integrase enzyme acts on the linear double-stranded viral DNA product of reverse transcription. Integrase cleaves specific phosphodiester bonds near the viral DNA ends during the 3' processing reaction. The enzyme then uses the resulting viral DNA 3'-OH groups during strand transfer to cut chromosomal target DNA, which simultaneously joins both viral DNA ends to target DNA 5'-phosphates. Both reactions proceed via direct transesterification of scissile phosphodiester bonds by attacking nucleophiles: a water molecule for 3' processing, and the viral DNA 3'-OH for strand transfer. X-ray crystal structures of prototype foamy virus integrase-DNA complexes revealed the architectures of the key nucleoprotein complexes that form sequentially during the integration process and explained the roles of active site metal ions in catalysis. X-ray crystallography furthermore elucidated the mechanism of action of HIV-1 integrase strand transfer inhibitors, which are currently used to treat AIDS patients, and provided valuable insights into the mechanisms of viral drug resistance.
Figures
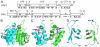


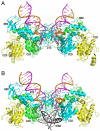

References
-
- Schwartzberg P, Colicelli J, Goff SP. Construction and analysis of deletion mutations in the pol gene of moloney murine leukemia virus: A new viral function required for productive infection. Cell. 1984;37:1043–1052. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

