The complex amino acid diet of Francisella in infected macrophages
- PMID: 25705612
- PMCID: PMC4319460
- DOI: 10.3389/fcimb.2015.00009
The complex amino acid diet of Francisella in infected macrophages
Abstract
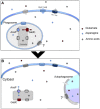
Francisella tularensis, the agent of the zoonotic disease tularemia, is a highly infectious bacterium for a large number of animal species and can be transmitted to humans by various means. The bacterium is able to infect a variety of cell types but replicates in mammalian hosts mainly in the cytosol of infected macrophages. In order to resist the stressful and nutrient-restricted intracellular environments, it encounters during its systemic dissemination, Francisella has developed dedicated stress resistance mechanisms and adapted its metabolic and nutritional needs. Recent data form our laboratory and from several other groups have shown that Francisella simultaneously relies on multiple host amino acid sources during its intracellular life cycle. This review will summarize how intracellular Francisella use different amino acid sources, and their role in phagosomal escape and/or cytosolic multiplication and systemic dissemination. We will first summarize the data that we have obtained on two amino acid transporters involved in Francisella phagosomal escape and cytosolic multiplication i.e., the glutamate transporter GadC and the asparagine transporter AnsP, respectively. The specific contribution of glutamate and asparagine to the physiology of the bacterium will be evoked. Then, we will discuss how Francisella has adapted to obtain and utilize host amino acid resources, and notably the contribution of host transporters and autophagy process in the establishment of a nutrient-replete intracellular niche.
Keywords: Francisella; amino acid uptake; intracellular pathogen; nutrition; phagosomal escape.
Figures
Similar articles
-
Importance of Metabolic Adaptations in Francisella Pathogenesis.Front Cell Infect Microbiol. 2017 Mar 28;7:96. doi: 10.3389/fcimb.2017.00096. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28401066 Free PMC article. Review.
-
Asparagine assimilation is critical for intracellular replication and dissemination of Francisella.Cell Microbiol. 2014 Mar;16(3):434-49. doi: 10.1111/cmi.12227. Epub 2013 Nov 8. Cell Microbiol. 2014. PMID: 24134488
-
Proteins involved in Francisella tularensis survival and replication inside macrophages.Future Microbiol. 2012 Nov;7(11):1255-68. doi: 10.2217/fmb.12.103. Future Microbiol. 2012. PMID: 23075445 Review.
-
Glutamate utilization couples oxidative stress defense and the tricarboxylic acid cycle in Francisella phagosomal escape.PLoS Pathog. 2014 Jan;10(1):e1003893. doi: 10.1371/journal.ppat.1003893. Epub 2014 Jan 16. PLoS Pathog. 2014. PMID: 24453979 Free PMC article.
-
A method for functional trans-complementation of intracellular Francisella tularensis.PLoS One. 2014 Feb 4;9(2):e88194. doi: 10.1371/journal.pone.0088194. eCollection 2014. PLoS One. 2014. PMID: 24505427 Free PMC article.
Cited by
-
How Viral and Intracellular Bacterial Pathogens Reprogram the Metabolism of Host Cells to Allow Their Intracellular Replication.Front Cell Infect Microbiol. 2019 Mar 4;9:42. doi: 10.3389/fcimb.2019.00042. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 30886834 Free PMC article. Review.
-
Proline utilization system is required for infection by the pathogenic α-proteobacterium Brucella abortus.Microbiology (Reading). 2017 Jul;163(7):970-979. doi: 10.1099/mic.0.000490. Epub 2017 Jul 21. Microbiology (Reading). 2017. PMID: 28691659 Free PMC article.
-
Dual RNA-Seq Uncovers Metabolic Amino Acids Dependency of the Intracellular Bacterium Piscirickettsia salmonis Infecting Atlantic Salmon.Front Microbiol. 2018 Nov 27;9:2877. doi: 10.3389/fmicb.2018.02877. eCollection 2018. Front Microbiol. 2018. PMID: 30542335 Free PMC article.
-
Manipulation of host membranes by the bacterial pathogens Listeria, Francisella, Shigella and Yersinia.Semin Cell Dev Biol. 2016 Dec;60:155-167. doi: 10.1016/j.semcdb.2016.07.019. Epub 2016 Jul 19. Semin Cell Dev Biol. 2016. PMID: 27448494 Free PMC article. Review.
-
To Eat and to Be Eaten: Mutual Metabolic Adaptations of Immune Cells and Intracellular Bacterial Pathogens upon Infection.Front Cell Infect Microbiol. 2017 Jul 13;7:316. doi: 10.3389/fcimb.2017.00316. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28752080 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

