Red blood cell destruction in autoimmune hemolytic anemia: role of complement and potential new targets for therapy
- PMID: 25705656
- PMCID: PMC4326213
- DOI: 10.1155/2015/363278
Red blood cell destruction in autoimmune hemolytic anemia: role of complement and potential new targets for therapy
Abstract
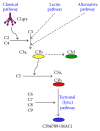
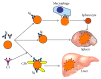
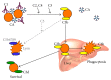
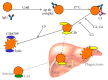
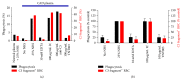
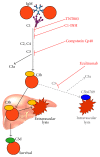
Autoimmune hemolytic anemia (AIHA) is a collective term for several diseases characterized by autoantibody-initiated destruction of red blood cells (RBCs). Exact subclassification is essential. We provide a review of the respective types of AIHA with emphasis on mechanisms of RBC destruction, focusing in particular on complement involvement. Complement activation plays a definitive but limited role in warm-antibody AIHA (w-AIHA), whereas primary cold agglutinin disease (CAD), secondary cold agglutinin syndrome (CAS), and paroxysmal cold hemoglobinuria (PCH) are entirely complement-dependent disorders. The details of complement involvement differ among these subtypes. The theoretical background for therapeutic complement inhibition in selected patients is very strong in CAD, CAS, and PCH but more limited in w-AIHA. The optimal target complement component for inhibition is assumed to be important and highly dependent on the type of AIHA. Complement modulation is currently not an evidence-based therapy modality in any AIHA, but a number of experimental and preclinical studies are in progress and a few clinical observations have been reported. Clinical studies of new complement inhibitors are probably not far ahead.
Figures
References
-
- Dacie J. The auto-immune haemolytic anaemias: introduction. In: Dacie J., editor. The Haemolytic Anaemias. Vol. 3. London, UK: Churchill Livingstone; 1992. pp. 1–5.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

