Requirement of cAMP signaling for Schwann cell differentiation restricts the onset of myelination
- PMID: 25705874
- PMCID: PMC4338006
- DOI: 10.1371/journal.pone.0116948
Requirement of cAMP signaling for Schwann cell differentiation restricts the onset of myelination
Abstract
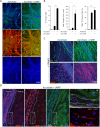
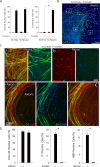
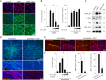
Isolated Schwann cells (SCs) respond to cAMP elevation by adopting a differentiated post-mitotic state that exhibits high levels of Krox-20, a transcriptional enhancer of myelination, and mature SC markers such as the myelin lipid galactocerebroside (O1). To address how cAMP controls myelination, we performed a series of cell culture experiments which compared the differentiating responses of isolated and axon-related SCs to cAMP analogs and ascorbate, a known inducer of axon ensheathment, basal lamina formation and myelination. In axon-related SCs, cAMP induced the expression of Krox-20 and O1 without a concomitant increase in the expression of myelin basic protein (MBP) and without promoting axon ensheathment, collagen synthesis or basal lamina assembly. When cAMP was provided together with ascorbate, a dramatic enhancement of MBP expression occurred, indicating that cAMP primes SCs to form myelin only under conditions supportive of basal lamina formation. Experiments using a combination of cell permeable cAMP analogs and type-selective adenylyl cyclase (AC) agonists and antagonists revealed that selective transmembrane AC (tmAC) activation with forskolin was not sufficient for full SC differentiation and that the attainment of an O1 positive state also relied on the activity of the soluble AC (sAC), a bicarbonate sensor that is insensitive to forskolin and GPCR activation. Pharmacological and immunological evidence indicated that SCs expressed sAC and that sAC activity was required for morphological differentiation and the expression of myelin markers such as O1 and protein zero. To conclude, our data indicates that cAMP did not directly drive myelination but rather the transition into an O1 positive state, which is perhaps the most critical cAMP-dependent rate limiting step for the onset of myelination. The temporally restricted role of cAMP in inducing differentiation independently of basal lamina formation provides a clear example of the uncoupling of signals controlling differentiation and myelination in SCs.
Conflict of interest statement
Figures










References
-
- Bunge MB, Clark MB, Dean AC, Eldridge CF, Bunge RP (1990) Schwann cell function depends upon axonal signals and basal lamina components. Ann N Y Acad Sci 580: 281–287. - PubMed
-
- Court FA, Wrabetz L, Feltri ML (2006) Basal lamina: Schwann cells wrap to the rhythm of space-time. Curr Opin Neurobiol 16: 501–507. - PubMed
-
- Topilko P, Schneider-Maunoury S, Levi G, Baron-Van Evercooren A, Chennoufi AB, et al. (1994) Krox-20 controls myelination in the peripheral nervous system. Nature 371: 796–799. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

