The bone conduction implant: Clinical results of the first six patients
- PMID: 25705995
- PMCID: PMC4487578
- DOI: 10.3109/14992027.2014.996826
The bone conduction implant: Clinical results of the first six patients
Abstract
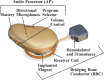
Objective: To investigate audiological and quality of life outcomes for a new active transcutaneous device, called the bone conduction implant (BCI), where the transducer is implanted under intact skin.
Design: A clinical study with sound field audiometry and questionnaires at six-month follow-up was conducted with a bone-anchored hearing aid on a softband as reference device.
Study sample: Six patients (age 18-67 years) with mild-to-moderate conductive or mixed hearing loss.
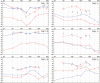
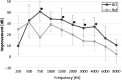
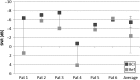
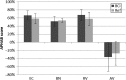
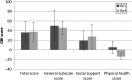
Results: The surgical procedure was found uneventful with no adverse events. The first hypothesis that BCI had a statistically significant improvement over the unaided condition was proven by a pure-tone-average improvement of 31.0 dB, a speech recognition threshold improvement in quiet (27.0 dB), and a speech recognition score improvement in noise (51.2 %). At speech levels, the signal-to-noise ratio threshold for BCI was - 5.5 dB. All BCI results were better than, or similar to the reference device results, and the APHAB and GBI questionnaires scores showed statistically significant improvements versus the unaided situation, supporting the second and third hypotheses.
Conclusions: The BCI provides significant hearing rehabilitation for patients with mild-to-moderate conductive or mixed hearing impairments, and can be easily and safely implanted under intact skin.
Keywords: Bone conduction implant; active transcutaneous; audiometry; hearing loss; intact skin; patient related outcome measure; questionnaires.
Figures
References
-
- Barbara M., Perotti M., Gioia B., Volpini L., Monini S. Transcutaneous bone-conduction hearing device: Audiological and surgical aspects in a first series of patients with mixed hearing loss. Acta Otolaryngol. 2013;133:1058–64. - PubMed
-
- Carlsson P., Håkansson B., Rosenhall U., Tjellström A. A speech-to-noise ratio test with the bone-anchored hearing aid: a comparative study. Otolaryngol Head Neck Surg. 1986;94:421–426. - PubMed
-
- Cochlear Bone Anchored Solutions celebrated 100 000 patients at the Osseo 2013 meeting; Newcastle, UK.
-
- Cox R.M., Alexander G.C. The abbreviated profile of hearing aid benefit. Ear Hear. 1995;16:176–186. - PubMed
-
- de Wolf M.J.F., Shival M.L.C., Hol M.K.S., Mylanus E.A.M., Cremers C.W.R.J., et al. Benefit and quality of life in older bone-anchored hearing aid users. Otol Neurotol. 2010;31:766–772. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
