Expression of the SNARE protein SNAP-23 is essential for cell survival
- PMID: 25706117
- PMCID: PMC4338070
- DOI: 10.1371/journal.pone.0118311
Expression of the SNARE protein SNAP-23 is essential for cell survival
Abstract
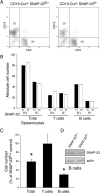
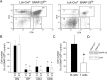
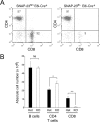
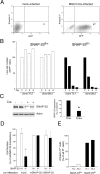
Members of the SNARE-family of proteins are known to be key regulators of the membrane-membrane fusion events required for intracellular membrane traffic. The ubiquitously expressed SNARE protein SNAP-23 regulates a wide variety of exocytosis events and is essential for mouse development. Germline deletion of SNAP-23 results in early embryonic lethality in mice, and for this reason we now describe mice and cell lines in which SNAP-23 can be conditionally-deleted using Cre-lox technology. Deletion of SNAP-23 in CD19-Cre expressing mice prevents B lymphocyte development and deletion of SNAP-23 using a variety of T lymphocyte-specific Cre mice prevents T lymphocyte development. Acute depletion of SNAP-23 in mouse fibroblasts leads to rapid apoptotic cell death. These data highlight the importance of SNAP-23 for cell survival and describe a mouse in which specific cell types can be eliminated by expression of tissue-specific Cre-recombinase.
Conflict of interest statement
Figures


References
-
- Jahn R, Scheller RH. SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol. 2006; 7: 631–643. - PubMed
-
- Washbourne P, Thompson PM, Carta M, Costa ET, Mathews JR, Lopez-Bendito G, et al. Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat Neurosci. 2002; 5: 19–26. - PubMed
-
- Foster LJ, Yaworsky K, Trimble WS, Klip A. SNAP23 promotes insulin-dependent glucose uptake in 3T3-L1 adipocytes: possible interaction with cytoskeleton. Am J Physiol. 1999; 276: C1108–1114. - PubMed
-
- Guo Z, Turner C, Castle D. Relocation of the t-SNARE SNAP-23 from lamellipodia-like cell surface projections regulates compound exocytosis in mast cells. Cell. 1998; 94: 537–548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

