Tracking early decline in cognitive function in older individuals at risk for Alzheimer disease dementia: the Alzheimer's Disease Cooperative Study Cognitive Function Instrument
- PMID: 25706191
- PMCID: PMC4397164
- DOI: 10.1001/jamaneurol.2014.3375
Tracking early decline in cognitive function in older individuals at risk for Alzheimer disease dementia: the Alzheimer's Disease Cooperative Study Cognitive Function Instrument
Erratum in
-
Error in figure legend.JAMA Neurol. 2015 May;72(5):608. doi: 10.1001/jamaneurol.2015.0382. JAMA Neurol. 2015. PMID: 25961182 No abstract available.
Abstract
Importance: Several large-scale Alzheimer disease (AD) secondary prevention trials have begun to target individuals at the preclinical stage. The success of these trials depends on validated outcome measures that are sensitive to early clinical progression in individuals who are initially asymptomatic.
Objective: To investigate the utility of the Cognitive Function Instrument (CFI) to track early changes in cognitive function in older individuals without clinical impairment at baseline.
Design, setting, and participants: Longitudinal study from February 2002 through February 2007 at participating Alzheimer's Disease Cooperative Study sites. Individuals were followed up annually for 48 months after the baseline visit. The study included 468 healthy older individuals (Clinical Dementia Rating scale [CDR] global scores of 0, above cutoff on the modified Mini-Mental State Examination and Free and Cued Selective Reminding Test) (mean [SD] age, 79.4 [3.6] years; age range, 75.0-93.8 years). All study participants and their study partners completed the self and partner CFIs annually. Individuals also underwent concurrent annual neuropsychological assessment and APOE genotyping.
Main outcomes and measures: The CFI scores between clinical progressors (CDR score, ≥0.5) and nonprogressors (CDR score, 0) and between APOE ε4 carriers and noncarriers were compared. Correlations of change between the CFI scores and neuropsychological performance were assessed longitudinally.
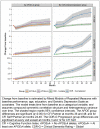
Results: At 48 months, group differences between clinical progressors and non-progressors were significant for self (2.13, SE=0.45, P<.001), partner (5.08, SE=0.59, P<.001), and self plus partner (7.04, SE=0.83, P<.001) CFI total scores. At month 48, APOE ε4 carriers had greater progression than noncarriers on the partner (1.10, SE=0.44, P<.012) and self plus partner (1.56, SE=0.63, P<.014) CFI scores. Both self and partner CFI change were associated with longitudinal cognitive decline (self, ρ=0.32, 95% CI, 0.13 to 0.46; partner, ρ=0.56, 95% CI, 0.42 to 0.68), although findings suggest self-report may be more accurate early in the process, whereas accuracy of partner report improves when there is progression to cognitive impairment.
Conclusions and relevance: Demonstrating long-term clinical benefit will be critical for the success of recently launched secondary prevention trials. The CFI appears to be a brief, but informative potential outcome measure that provides insight into functional abilities at the earliest stages of disease.
Figures

References
-
- Guidance for industry: Alzheimer's disease: developing drugs for the treatment of early stage disease (Draft Guidance) United States Food and Drug Administration; Silver Spring, MD: 2013.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

