SATB1 overexpression regulates the development and progression in bladder cancer through EMT
- PMID: 25706386
- PMCID: PMC4338074
- DOI: 10.1371/journal.pone.0117518
SATB1 overexpression regulates the development and progression in bladder cancer through EMT
Retraction in
-
Retraction: SATB1 Overexpression Regulates the Development and Progression in Bladder Cancer through EMT.PLoS One. 2024 Apr 2;19(4):e0301572. doi: 10.1371/journal.pone.0301572. eCollection 2024. PLoS One. 2024. PMID: 38564501 Free PMC article. No abstract available.
Abstract
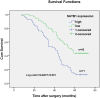
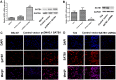
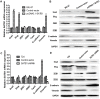
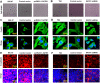
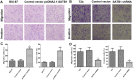
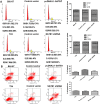
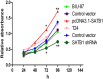
The global gene regulator Special AT-rich sequence-binding protein-1 (SATB1) has been reported to induce EMT-like changes and be associated with poor clinical outcome in several cancers. This study aims to evaluate whether SATB1 affects the biological behaviors of bladder transitional cell carcinoma (BTCC) and further elucidate if this effect works through an epithelial-mesenchymal transition (EMT) pathway. The expression of SATB1, E-cadherin (epithelial markers), vimentin (mesenchymal markers) in BTCC tissues and adjacent noncancerous tissues, as well as in two cell lines of bladder cancer were investigated. Whether the SATB1 expression is associated with clinicopathological factors or not was statistically analyzed. Cell invasion and migration, cell cycle, cell proliferation and apoptosis were evaluated in SATB1 knockdown and overexpressed cell lines. Our results showed that the expression of SATB1 was remarkably up-regulated both in BTCC tissues and in bladder cancer cell lines with high potential of metastasis. The results were also associated with EMT markers and poor prognosis of BTCC patients. Moreover, SATB1 induced EMT processes through downregulation of E-cadherin, upregulation of E-cadherin repressors (Snail, Slug and vimentin). SATB1 also promoted cell cycle progression, cell proliferation, cell invasion and cell migration, but did not alter cell survival. In conclusion, our results suggest that SATB1 plays a crucial role in the progression of bladder cancer by regulating genes controlling EMT processes. Further, it may be a novel therapeutic target for aggressive bladder cancers.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

