Influenza A virus infection of intestinal epithelial cells enhances the adhesion ability of Crohn's disease associated Escherichia coli strains
- PMID: 25706391
- PMCID: PMC4338238
- DOI: 10.1371/journal.pone.0117005
Influenza A virus infection of intestinal epithelial cells enhances the adhesion ability of Crohn's disease associated Escherichia coli strains
Abstract
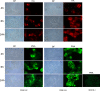
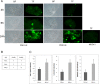
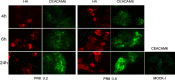
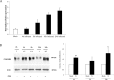
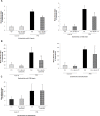
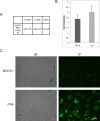
Modifications of intestinal glycoreceptors expression, in particular CEACAM6, typically found in ileal Crohn's disease (CD), favor, among the commensal species of microbiota, the enrichment in Escherichia coli. Removal of protein glycosidic residues by neuraminidase, a sialidase typical of influenza virus, increases adhesion ability of Escherichia coli to Caco-2 intestinal cells. In this study we investigated whether influenza virus infection of human intestinal epithelial cells could influence the adhesiveness of different Escherichia coli strains isolated from CD patients by altering surface glycoreceptors. Influenza virus infection of intestinal cells increased exposure of galactose and mannose residues on the cell surface. In particular, glycoreceptors Thomsen-Friedenreich and CEACAM6 were over-expressed in influenza virus infected cells. In the same experimental conditions, a significant increase in bacterial adhesiveness was observed, independently of their own adhesive ability. The increase was reverted by treatment with anti-TF and anti-CEACAM6 antibodies. Interestingly, influenza virus was able to efficiently replicate in human primary intestinal cells leading to TF exposure. Finally, intestinal infected cells produced high levels of pro-inflammatory cytokines compared to control. Overall these data suggest that influenza virus infection, could constitute an additional risk factor in CD patients.
Conflict of interest statement
Figures

References
-
- Xavier RJ, Podolsky DK (2007) Unravelling the pathogenesis of inflammatory bowel disease. Nature 448: 427–434. - PubMed
-
- Podolsky DK (1997) Lessons from genetic models of inflammatory bowel disease. Acta Gastroenterol Belg 60:163–165. - PubMed
-
- Cario E, Gerken G, Podolsky DK (2007) Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 132: 1359–1374. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

