A subcortical inhibitory signal for behavioral arrest in the thalamus
- PMID: 25706472
- PMCID: PMC4885661
- DOI: 10.1038/nn.3951
A subcortical inhibitory signal for behavioral arrest in the thalamus
Abstract
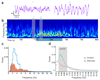
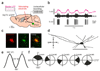
Organization of behavior requires rapid coordination of brainstem and forebrain activity. The exact mechanisms of effective communication between these regions are presently unclear. The intralaminar thalamic nuclei (IL) probably serves as a central hub in this circuit by connecting the critical brainstem and forebrain areas. We found that GABAergic and glycinergic fibers ascending from the pontine reticular formation (PRF) of the brainstem evoked fast and reliable inhibition in the IL via large, multisynaptic terminals. This inhibition was fine-tuned through heterogeneous GABAergic and glycinergic receptor ratios expressed at individual synapses. Optogenetic activation of PRF axons in the IL of freely moving mice led to behavioral arrest and transient interruption of awake cortical activity. An afferent system with comparable morphological features was also found in the human IL. These data reveal an evolutionarily conserved ascending system that gates forebrain activity through fast and powerful synaptic inhibition of the IL.
Figures




References
-
- Drew T, Andujar J-E, Lajoie K, Yakovenko S. Cortical mechanisms involved in visuomotor coordination during precision walking. Brain Res Rev. 2008;57:199–211. - PubMed
-
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. - PubMed
-
- Rizzolatti G, Luppino G. The cortical motor system. Neuron. 2001;31:889–901. - PubMed
-
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

