What does gamma coherence tell us about inter-regional neural communication?
- PMID: 25706474
- PMCID: PMC4803441
- DOI: 10.1038/nn.3952
What does gamma coherence tell us about inter-regional neural communication?
Abstract
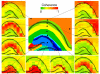
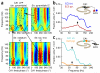
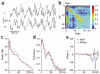
Neural oscillations have been measured and interpreted in multitudinous ways, with a variety of hypothesized functions in physiology, information processing and cognition. Much attention has been paid in recent years to gamma-band (30-100 Hz) oscillations and synchrony, with an increasing interest in 'high gamma' (>100 Hz) signals as mesoscopic measures of inter-regional communication. The biophysical origins of the measured variables are often difficult to precisely identify, however, making their interpretation fraught with pitfalls. Here we discuss how measurements of inter-regional gamma coherence can be prone to misinterpretation and suggest strategies for deciphering the roles that synchronized oscillations across brain networks may play in neural function.
Figures
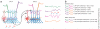

Similar articles
-
Gamma coherence mediates interhemispheric integration during multiple object tracking.J Neurophysiol. 2020 May 1;123(5):1630-1644. doi: 10.1152/jn.00755.2019. Epub 2020 Mar 18. J Neurophysiol. 2020. PMID: 32186427
-
Communication between Brain Areas Based on Nested Oscillations.eNeuro. 2017 Mar 27;4(2):ENEURO.0153-16.2017. doi: 10.1523/ENEURO.0153-16.2017. eCollection 2017 Mar-Apr. eNeuro. 2017. PMID: 28374013 Free PMC article.
-
Flexible information routing by transient synchrony.Nat Neurosci. 2017 Jul;20(7):1014-1022. doi: 10.1038/nn.4569. Epub 2017 May 22. Nat Neurosci. 2017. PMID: 28530664
-
Functional integration across oscillation frequencies by cross-frequency phase synchronization.Eur J Neurosci. 2018 Oct;48(7):2399-2406. doi: 10.1111/ejn.13767. Epub 2017 Dec 2. Eur J Neurosci. 2018. PMID: 29094462 Review.
-
Functions of gamma-band synchronization in cognition: from single circuits to functional diversity across cortical and subcortical systems.Eur J Neurosci. 2014 Jun;39(11):1982-99. doi: 10.1111/ejn.12606. Epub 2014 May 8. Eur J Neurosci. 2014. PMID: 24809619 Review.
Cited by
-
Concomitant Processing of Choice and Outcome in Frontal Corticostriatal Ensembles Correlates with Performance of Rats.Cereb Cortex. 2021 Jul 29;31(9):4357-4375. doi: 10.1093/cercor/bhab091. Cereb Cortex. 2021. PMID: 33914862 Free PMC article.
-
Decreased electrocortical temporal complexity distinguishes sleep from wakefulness.Sci Rep. 2019 Dec 5;9(1):18457. doi: 10.1038/s41598-019-54788-6. Sci Rep. 2019. PMID: 31804569 Free PMC article.
-
Neurophysiological assessment of cortical activity in DEPDC5- and NPRL3-related epileptic mTORopathies.Orphanet J Rare Dis. 2023 Jan 14;18(1):11. doi: 10.1186/s13023-022-02600-6. Orphanet J Rare Dis. 2023. PMID: 36639812 Free PMC article.
-
TMS-EEG Biomarkers of Amnestic Mild Cognitive Impairment Due to Alzheimer's Disease: A Proof-of-Concept Six Years Prospective Study.Front Aging Neurosci. 2021 Nov 22;13:737281. doi: 10.3389/fnagi.2021.737281. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34880743 Free PMC article.
-
Age-related decline in cortical inhibitory tone strengthens motor memory.Neuroimage. 2021 Dec 15;245:118681. doi: 10.1016/j.neuroimage.2021.118681. Epub 2021 Oct 30. Neuroimage. 2021. PMID: 34728243 Free PMC article.
References
-
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat. Rev. Neurosci. 2001;2:229–239. - PubMed
-
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top–down processing. Nat. Rev. Neurosci. 2001;2:704–716. - PubMed
-
- Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

