Neuronal ensembles sufficient for recovery sleep and the sedative actions of α2 adrenergic agonists
- PMID: 25706476
- PMCID: PMC4836567
- DOI: 10.1038/nn.3957
Neuronal ensembles sufficient for recovery sleep and the sedative actions of α2 adrenergic agonists
Abstract
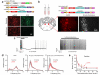
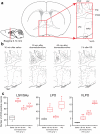
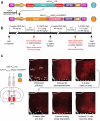
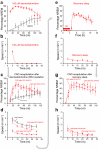
Do sedatives engage natural sleep pathways? It is usually assumed that anesthetic-induced sedation and loss of righting reflex (LORR) arise by influencing the same circuitry to lesser or greater extents. For the α2 adrenergic receptor agonist dexmedetomidine, we found that sedation and LORR were in fact distinct states, requiring different brain areas: the preoptic hypothalamic area and locus coeruleus (LC), respectively. Selective knockdown of α2A adrenergic receptors from the LC abolished dexmedetomidine-induced LORR, but not sedation. Instead, we found that dexmedetomidine-induced sedation resembled the deep recovery sleep that follows sleep deprivation. We used TetTag pharmacogenetics in mice to functionally mark neurons activated in the preoptic hypothalamus during dexmedetomidine-induced sedation or recovery sleep. The neuronal ensembles could then be selectively reactivated. In both cases, non-rapid eye movement sleep, with the accompanying drop in body temperature, was recapitulated. Thus, α2 adrenergic receptor-induced sedation and recovery sleep share hypothalamic circuitry sufficient for producing these behavioral states.
Figures

Similar articles
-
Galanin Neurons Unite Sleep Homeostasis and α2-Adrenergic Sedation.Curr Biol. 2019 Oct 7;29(19):3315-3322.e3. doi: 10.1016/j.cub.2019.07.087. Epub 2019 Sep 19. Curr Biol. 2019. PMID: 31543455 Free PMC article.
-
The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects.Anesthesiology. 2003 Feb;98(2):428-36. doi: 10.1097/00000542-200302000-00024. Anesthesiology. 2003. PMID: 12552203
-
Staying awake--a genetic region that hinders α2 adrenergic receptor agonist-induced sleep.Eur J Neurosci. 2014 Jul;40(1):2311-9. doi: 10.1111/ejn.12570. Epub 2014 Mar 27. Eur J Neurosci. 2014. PMID: 24674448 Free PMC article.
-
Sleep and Sedative States Induced by Targeting the Histamine and Noradrenergic Systems.Front Neural Circuits. 2018 Jan 26;12:4. doi: 10.3389/fncir.2018.00004. eCollection 2018. Front Neural Circuits. 2018. PMID: 29434539 Free PMC article. Review.
-
[Dexmedetomidine hydrochloride (Precedex), a new sedative in intensive care, its pharmacological characteristics and clinical study result].Nihon Yakurigaku Zasshi. 2004 Sep;124(3):171-9. doi: 10.1254/fpj.124.171. Nihon Yakurigaku Zasshi. 2004. PMID: 15333990 Review. Japanese.
Cited by
-
A noradrenergic-hypothalamic neural substrate for stress-induced sleep disturbances.Proc Natl Acad Sci U S A. 2022 Nov 8;119(45):e2123528119. doi: 10.1073/pnas.2123528119. Epub 2022 Nov 4. Proc Natl Acad Sci U S A. 2022. PMID: 36331996 Free PMC article.
-
Auditory Predictive Coding across Awareness States under Anesthesia: An Intracranial Electrophysiology Study.J Neurosci. 2018 Sep 26;38(39):8441-8452. doi: 10.1523/JNEUROSCI.0967-18.2018. Epub 2018 Aug 20. J Neurosci. 2018. PMID: 30126970 Free PMC article.
-
Comparison of prefrontal cortex sucrose seeking ensembles engaged in multiple seeking sessions: Context is key.J Neurosci Res. 2022 Apr;100(4):1008-1029. doi: 10.1002/jnr.25025. Epub 2022 Feb 9. J Neurosci Res. 2022. PMID: 35137974 Free PMC article.
-
Complementary Genetic Targeting and Monosynaptic Input Mapping Reveal Recruitment and Refinement of Distributed Corticostriatal Ensembles by Cocaine.Neuron. 2019 Dec 4;104(5):916-930.e5. doi: 10.1016/j.neuron.2019.10.032. Epub 2019 Nov 20. Neuron. 2019. PMID: 31759807 Free PMC article.
-
Herpes zoster chronification to postherpetic neuralgia induces brain activity and grey matter volume change.Am J Transl Res. 2018 Jan 15;10(1):184-199. eCollection 2018. Am J Transl Res. 2018. PMID: 29423004 Free PMC article.
References
-
- Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nature reviews. Neuroscience. 2008;9:370–386. - PubMed
-
- Adams R, et al. Efficacy of dexmedetomidine compared with midazolam for sedation in adult intensive care patients: a systematic review. British journal of anaesthesia. 2013;111:703–710. - PubMed
-
- Bol C, Danhof M, Stanski DR, Mandema JW. Pharmacokinetic-pharmacodynamic characterization of the cardiovascular, hypnotic, EEG and ventilatory responses to dexmedetomidine in the rat. The Journal of pharmacology and experimental therapeutics. 1997;283:1051–1058. - PubMed
-
- Seidel WF, Maze M, Dement WC, Edgar DM. Alpha-2 adrenergic modulation of sleep: time-of-day-dependent pharmacodynamic profiles of dexmedetomidine and clonidine in the rat. The Journal of pharmacology and experimental therapeutics. 1995;275:263–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/F017324/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/K018159/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G021691/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- WT094211/WT_/Wellcome Trust/United Kingdom
- G0901892/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

