Two classes of bacterial IMPDHs according to their quaternary structures and catalytic properties
- PMID: 25706619
- PMCID: PMC4338043
- DOI: 10.1371/journal.pone.0116578
Two classes of bacterial IMPDHs according to their quaternary structures and catalytic properties
Erratum in
-
Correction: Two Classes of Bacterial IMPDHs according to Their Quaternary Structures and Catalytic Properties.PLoS One. 2015 Apr 29;10(4):e0125884. doi: 10.1371/journal.pone.0125884. eCollection 2015. PLoS One. 2015. PMID: 25923221 Free PMC article. No abstract available.
Abstract
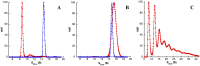
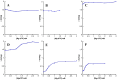
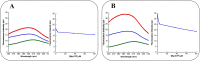
Inosine-5'-monophosphate dehydrogenase (IMPDH) occupies a key position in purine nucleotide metabolism. In this study, we have performed the biochemical and physico-chemical characterization of eight bacterial IMPDHs, among which six were totally unexplored. This study led to a classification of bacterial IMPDHs according to the regulation of their catalytic properties and their quaternary structures. Class I IMPDHs are cooperative enzymes for IMP, which are activated by MgATP and are octameric in all tested conditions. On the other hand, class II IMPDHs behave as Michaelis-Menten enzymes for both substrates and are tetramers in their apo state or in the presence of IMP, which are shifted to octamers in the presence of NAD or MgATP. Our work provides new insights into the IMPDH functional regulation and a model for the quaternary structure modulation is proposed.
Conflict of interest statement
Figures




Similar articles
-
MgATP regulates allostery and fiber formation in IMPDHs.Structure. 2013 Jun 4;21(6):975-85. doi: 10.1016/j.str.2013.03.011. Epub 2013 May 2. Structure. 2013. PMID: 23643948
-
Insight into the role of the Bateman domain at the molecular and physiological levels through engineered IMP dehydrogenases.Protein Sci. 2023 Aug;32(8):e4703. doi: 10.1002/pro.4703. Protein Sci. 2023. PMID: 37338125 Free PMC article.
-
A novel cofactor-binding mode in bacterial IMP dehydrogenases explains inhibitor selectivity.J Biol Chem. 2015 Feb 27;290(9):5893-911. doi: 10.1074/jbc.M114.619767. Epub 2015 Jan 8. J Biol Chem. 2015. PMID: 25572472 Free PMC article.
-
Inosine monophosphate dehydrogenase (IMPDH) as a target in drug discovery.Med Res Rev. 2008 Mar;28(2):219-32. doi: 10.1002/med.20104. Med Res Rev. 2008. PMID: 17480004 Review.
-
The antibiotic potential of prokaryotic IMP dehydrogenase inhibitors.Curr Med Chem. 2011;18(13):1909-18. doi: 10.2174/092986711795590129. Curr Med Chem. 2011. PMID: 21517780 Free PMC article. Review.
Cited by
-
Correction: Two Classes of Bacterial IMPDHs according to Their Quaternary Structures and Catalytic Properties.PLoS One. 2015 Apr 29;10(4):e0125884. doi: 10.1371/journal.pone.0125884. eCollection 2015. PLoS One. 2015. PMID: 25923221 Free PMC article. No abstract available.
-
Guanine nucleotide binding to the Bateman domain mediates the allosteric inhibition of eukaryotic IMP dehydrogenases.Nat Commun. 2015 Nov 12;6:8923. doi: 10.1038/ncomms9923. Nat Commun. 2015. PMID: 26558346 Free PMC article.
-
In cellulo crystallization of Trypanosoma brucei IMP dehydrogenase enables the identification of genuine co-factors.Nat Commun. 2020 Jan 30;11(1):620. doi: 10.1038/s41467-020-14484-w. Nat Commun. 2020. PMID: 32001697 Free PMC article.
-
Expanding Benzoxazole-Based Inosine 5'-Monophosphate Dehydrogenase (IMPDH) Inhibitor Structure-Activity As Potential Antituberculosis Agents.J Med Chem. 2018 Jun 14;61(11):4739-4756. doi: 10.1021/acs.jmedchem.7b01839. Epub 2018 May 30. J Med Chem. 2018. PMID: 29746130 Free PMC article.
-
Transcription of IVIAT and Virulence Genes in Photobacterium damselae Subsp. piscicida Infecting Solea senegalensis.Microorganisms. 2018 Jul 12;6(3):67. doi: 10.3390/microorganisms6030067. Microorganisms. 2018. PMID: 30002314 Free PMC article.
References
-
- (2013) Allosteric Interactions and Biological Regulation (Part II). J Mol Biol 425: 2227–2392. - PubMed
-
- Monod J, Wyman J, Changeux JP (1965) On the Nature of Allosteric Transitions: a Plausible Model. J Mol Biol 12: 88–118. - PubMed
-
- Koshland DE Jr, Nemethy G, Filmer D (1966) Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry 5: 365–385. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

