Anticancer properties of lamellarins
- PMID: 25706633
- PMCID: PMC4377975
- DOI: 10.3390/md13031105
Anticancer properties of lamellarins
Abstract
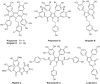
In 1985 the first lamellarins were isolated from a small oceanic sea snail. Today, more than 50 lamellarins have been inventoried and numerous derivatives synthesized and tested as antiviral or anticancer agents. The lead compound in the family is lamellarin D, characterized as a potent inhibitor of both nuclear and mitochondrial topoisomerase I but also capable of directly interfering with mitochondria to trigger cancer cell death. The pharmacology and chemistry of lamellarins are discussed here and the mechanistic portrait of lamellarin D is detailed. Lamellarins frequently serve as a starting point in the design of anticancer compounds. Extensive efforts have been devoted to create novel structures as well as to improve synthetic methods, leading to lamellarins and related pyrrole-derived marine alkaloids.
Figures




References
-
- Cavalcanti B.C., Júnior H.V., Seleghim M.H., Berlinck R.G., Cunha G.M., Moraes M.O., Pessoa C. Cytotoxic and genotoxic effects of tambjamine D, an alkaloid isolated from the nudibranch Tambja eliora, on Chinese hamster lung fibroblasts. Chem. Biol. Interact. 2008;174:155–162. doi: 10.1016/j.cbi.2008.05.029. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

