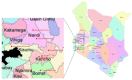
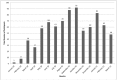
Kericho CLinic-based ART Diagnostic Evaluation (CLADE): design, accrual, and baseline characteristics of a randomized controlled trial conducted in predominately rural, district-level, HIV clinics of Kenya
- PMID: 25706652
- PMCID: PMC4338154
- DOI: 10.1371/journal.pone.0116299
Kericho CLinic-based ART Diagnostic Evaluation (CLADE): design, accrual, and baseline characteristics of a randomized controlled trial conducted in predominately rural, district-level, HIV clinics of Kenya
Abstract
Background: Prospective clinical trial data regarding routine HIV-1 viral load (VL) monitoring of antiretroviral therapy (ART) in non-research clinics of Sub-Saharan Africa are needed for policy makers.
Methods: CLinic-based ART Diagnostic Evaluation (CLADE) is a randomized, controlled trial (RCT) evaluating feasibility, superiority, and cost-effectiveness of routine VL vs. standard of care (clinical and immunological) monitoring in adults initiating dual nucleoside reverse transcriptase inhibitor (NRTI)+non-NRTI ART. Participants were randomized (1:1) at 7 predominately rural, non-research, district-level clinics of western Kenya. Descriptive statistics present accrual patterns and baseline cohort characteristics.
Results: Over 15 months, 820 adults enrolled at 7 sites with 86-152 enrolled per site. Monthly site enrollment ranged from 2-92 participants. Full (100%) informed consent compliance was independently documented. Half (49.9%) had HIV diagnosed through voluntary counseling and testing. Study arms were similar: mostly females (57.6%) aged 37.6 (SD = 9.0) years with low CD4 (166 [SD = 106]) cells/m3). Notable proportions had WHO Stage III or IV disease (28.7%), BMI <18.5 kg/m2 (23.1%), and a history of tuberculosis (5.6%) or were receiving tuberculosis treatment (8.2%) at ART initiation. In the routine VL arm, 407/409 (99.5%) received baseline VL (234,577 SD = 151,055 copies/ml). All participants received lamivudine; 49.8% started zidovudine followed by 38.4% stavudine and 11.8% tenofovir; and, 64.4% received nevirapine as nNRTI (35.6% efavirenz).
Conclusions: A RCT can be enrolled successfully in rural, non-research, resource limited, district-level clinics in western Kenya. Many adults presenting for ART have advanced HIV/AIDS, emphasizing the importance of universal HIV testing and linkage-to-care campaigns.
Trial registration: ClinicalTrials.gov NCT01791556.
Conflict of interest statement
Figures
References
-
- Department of Health and Human Services, Panel on Antiretroviral Guidelines for Adults and Adolescents (2012) Guidelines for the use of antiretroviral agents in HIV-1-infected Adults and Adolescents; Washington (DC): Department of Health and Human Services; Available: http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed January 16, 2013.
-
- European AIDS Clinical Society (EACS). Guidelines v6.1 November 2012. Available: http://www.eacsociety.org/Portals/0/files/pdf%20files/EacsGuidelines-v6....
-
- World Health Organization (WHO) (2006) Antiretroviral Thearpy for HIV Infection in Adults and Adolscents in Resource Limited Settings: Towards Universal Access Recommendations for a Public Health Approach 2006 Revision. Available: http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf?ua = 1
-
- Eisenhut M (2008) Causes for low positive predictive values of CD4 counts for antiretroviral treatment failure. Int J Infect Dis 12(5):565; author reply 566. doi: 10.1016/j.ijid.2008.02.002. M Epub 2008 Apr 9 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials