(89)Zr-Oxine Complex PET Cell Imaging in Monitoring Cell-based Therapies
- PMID: 25706654
- PMCID: PMC4456181
- DOI: 10.1148/radiol.15142849
(89)Zr-Oxine Complex PET Cell Imaging in Monitoring Cell-based Therapies
Abstract
Purpose: To develop a clinically translatable method of cell labeling with zirconium 89 ((89)Zr) and oxine to track cells with positron emission tomography (PET) in mouse models of cell-based therapy.
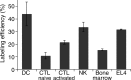
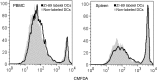
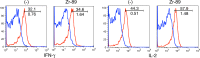
Materials and methods: This study was approved by the institutional animal care committee. (89)Zr-oxine complex was synthesized in an aqueous solution. Cell labeling conditions were optimized by using EL4 mouse lymphoma cells, and labeling efficiency was examined by using dendritic cells (DCs) (n = 4), naïve (n = 3) and activated (n = 3) cytotoxic T cells (CTLs), and natural killer (NK) (n = 4), bone marrow (n = 4), and EL4 (n = 4) cells. The effect of (89)Zr labeling on cell survival, proliferation, and function were evaluated by using DCs (n = 3) and CTLs (n = 3). Labeled DCs (444-555 kBq/[5 × 10(6)] cells, n = 5) and CTLs (185 kBq/[5 × 10(6)] cells, n = 3) transferred to mice were tracked with microPET/CT. In a melanoma immunotherapy model, tumor targeting and cytotoxic function of labeled CTLs were evaluated with imaging (248.5 kBq/[7.7 × 10(6)] cells, n = 4) and by measuring the tumor size (n = 6). Two-way analysis of variance was used to compare labeling conditions, the Wilcoxon test was used to assess cell survival and proliferation, and Holm-Sidak multiple tests were used to assess tumor growth and perform biodistribution analyses.
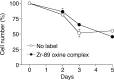
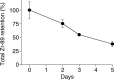
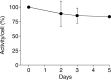
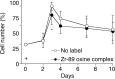
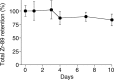
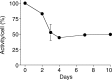
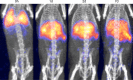
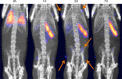
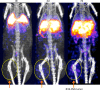
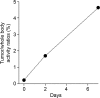
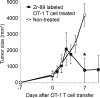
Results: (89)Zr-oxine complex was synthesized at a mean yield of 97.3% ± 2.8 (standard deviation). It readily labeled cells at room temperature or 4°C in phosphate-buffered saline (labeling efficiency range, 13.0%-43.9%) and was stably retained (83.5% ± 1.8 retention on day 5 in DCs). Labeling did not affect the viability of DCs and CTLs when compared with nonlabeled control mice (P > .05), nor did it affect functionality. (89)Zr-oxine complex enabled extended cell tracking for 7 days. Labeled tumor-specific CTLs accumulated in the tumor (4.6% on day 7) and induced tumor regression (P < .05 on day 7).
Conclusion: We have developed a (89)Zr-oxine complex cell tracking technique for use with PET that is applicable to a broad range of cell types and could be a valuable tool with which to evaluate various cell-based therapies.
(©) RSNA, 2015
Figures

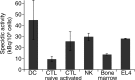


Similar articles
-
Tracking of NK Cells by Positron Emission Tomography Using 89Zr-Oxine Ex Vivo Cell Labeling.Methods Mol Biol. 2022;2463:153-161. doi: 10.1007/978-1-0716-2160-8_11. Methods Mol Biol. 2022. PMID: 35344173
-
In Vivo Tracking of Adoptively Transferred Natural Killer Cells in Rhesus Macaques Using 89Zirconium-Oxine Cell Labeling and PET Imaging.Clin Cancer Res. 2020 Jun 1;26(11):2573-2581. doi: 10.1158/1078-0432.CCR-19-2897. Epub 2020 Feb 7. Clin Cancer Res. 2020. PMID: 32034075 Free PMC article.
-
Bone Marrow Cell Trafficking Analyzed by 89Zr-oxine Positron Emission Tomography in a Murine Transplantation Model.Clin Cancer Res. 2017 Jun 1;23(11):2759-2768. doi: 10.1158/1078-0432.CCR-16-1561. Epub 2016 Dec 13. Clin Cancer Res. 2017. PMID: 27965305 Free PMC article.
-
A kit formulation for the preparation of [89Zr]Zr(oxinate)4 for PET cell tracking: White blood cell labelling and comparison with [111In]In(oxinate)3.Nucl Med Biol. 2020 Nov-Dec;90-91:31-40. doi: 10.1016/j.nucmedbio.2020.09.002. Epub 2020 Sep 15. Nucl Med Biol. 2020. PMID: 32979725 Free PMC article.
-
Imaging of cell-based therapy using 89Zr-oxine ex vivo cell labeling for positron emission tomography.Nanotheranostics. 2021 Jan 1;5(1):27-35. doi: 10.7150/ntno.51391. eCollection 2021. Nanotheranostics. 2021. PMID: 33391973 Free PMC article. Review.
Cited by
-
In vivo Imaging Technologies to Monitor the Immune System.Front Immunol. 2020 Jun 2;11:1067. doi: 10.3389/fimmu.2020.01067. eCollection 2020. Front Immunol. 2020. PMID: 32582173 Free PMC article. Review.
-
Glucocorticoid-induced eosinopenia results from CXCR4-dependent bone marrow migration.Blood. 2020 Dec 3;136(23):2667-2678. doi: 10.1182/blood.2020005161. Blood. 2020. PMID: 32659786 Free PMC article. Clinical Trial.
-
Total-Body PET and Highly Stable Chelators Together Enable Meaningful 89Zr-Antibody PET Studies up to 30 Days After Injection.J Nucl Med. 2020 Mar;61(3):453-460. doi: 10.2967/jnumed.119.230961. Epub 2019 Sep 27. J Nucl Med. 2020. PMID: 31562219 Free PMC article.
-
Direct Cell Radiolabeling for in Vivo Cell Tracking with PET and SPECT Imaging.Chem Rev. 2022 Jun 8;122(11):10266-10318. doi: 10.1021/acs.chemrev.1c00767. Epub 2022 May 12. Chem Rev. 2022. PMID: 35549242 Free PMC article. Review.
-
Preparation of Zirconium-89 Solutions for Radiopharmaceutical Purposes: Interrelation Between Formulation, Radiochemical Purity, Stability and Biodistribution.Molecules. 2019 Apr 18;24(8):1534. doi: 10.3390/molecules24081534. Molecules. 2019. PMID: 31003494 Free PMC article.
References
-
- O’Neill DW, Adams S, Bhardwaj N. Manipulating dendritic cell biology for the active immunotherapy of cancer. Blood 2004;104(8):2235–2246. - PubMed
-
- Kater AP, van Oers MH, Kipps TJ. Cellular immune therapy for chronic lymphocytic leukemia. Blood 2007;110(8):2811–2818. - PubMed
-
- Körbling M, Freireich EJ. Twenty-five years of peripheral blood stem cell transplantation. Blood 2011;117(24):6411–6416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

