Anti-glycation activities of phenolic constituents from Silybum marianum (Milk Thistle) flower in vitro and on human explants
- PMID: 25706757
- PMCID: PMC6272457
- DOI: 10.3390/molecules20033549
Anti-glycation activities of phenolic constituents from Silybum marianum (Milk Thistle) flower in vitro and on human explants
Abstract
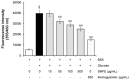
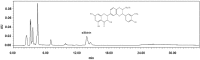
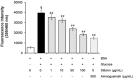
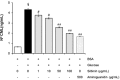
Glycation is an ageing reaction of naturally occurring sugars with dermal proteins, with clinical signs appearing in vivo around age 30, and increasing steadily/regularly with age. The suppleness of the dermis is affected by the formation of bridges between proteins and sugars (Maillard's reaction). The accumulation of advanced glycation end products (AGEs) in skin plays a very important role in skin ageing. Therefore, natural compounds or extracts that possess antiglycation activities may have great anti-ageing potential. In the present study, Silybum marianum flower extract (SMFE) was demonstrated to possess antiglycation activity. We found that SMFE inhibits glycation reaction between BSA and glucose. In addition, antiglycation activity of SMFE was confirmed in a human skin explants model. SMFE reduced Nε-(carboxymethyl) lysine (CML) expression, whereas SMFE stimulated fibrillin-1 expression compared to treatment with methyglyoxal. An active ingredient contributing to the observed activities was identified as silibinin. The antiglycation activity of silibinin was dose-dependent. The beneficial effects of silibinin may be applied to prevention or management of AGE-mediated pathologies, targeting in a pleiotropic and complementary way the biochemical and cellular bases of skin aging.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Ameliorating Effect of Akebia quinata Fruit Extracts on Skin Aging Induced by Advanced Glycation End Products.Nutrients. 2015 Nov 12;7(11):9337-52. doi: 10.3390/nu7115478. Nutrients. 2015. PMID: 26569300 Free PMC article.
-
Glycation induction and antiglycation activity of skin care ingredients on living human skin explants.Int J Cosmet Sci. 2011 Aug;33(4):366-70. doi: 10.1111/j.1468-2494.2011.00640.x. Epub 2011 Mar 15. Int J Cosmet Sci. 2011. PMID: 21401648
-
Senotherapeutic-like effect of Silybum marianum flower extract revealed on human skin cells.PLoS One. 2021 Dec 16;16(12):e0260545. doi: 10.1371/journal.pone.0260545. eCollection 2021. PLoS One. 2021. PMID: 34914725 Free PMC article.
-
Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)-Chemistry, Bioavailability, and Metabolism.Molecules. 2017 Nov 10;22(11):1942. doi: 10.3390/molecules22111942. Molecules. 2017. PMID: 29125572 Free PMC article. Review.
-
Silybum marianum (milk thistle) and its main constituent, silymarin, as a potential therapeutic plant in metabolic syndrome: A review.Phytother Res. 2018 Oct;32(10):1933-1949. doi: 10.1002/ptr.6153. Epub 2018 Jul 17. Phytother Res. 2018. PMID: 30015401 Review.
Cited by
-
Research Advances on the Damage Mechanism of Skin Glycation and Related Inhibitors.Nutrients. 2022 Nov 1;14(21):4588. doi: 10.3390/nu14214588. Nutrients. 2022. PMID: 36364850 Free PMC article. Review.
-
Selective Synthesis of 3-O-Palmitoyl-Silybin, a New-to-Nature Flavonolignan with Increased Protective Action against Oxidative Damages in Lipophilic Media.Molecules. 2018 Oct 10;23(10):2594. doi: 10.3390/molecules23102594. Molecules. 2018. PMID: 30309022 Free PMC article.
-
Recent Advances in Traditional Chinese Medicine for Treatment of Podocyte Injury.Front Pharmacol. 2022 Feb 25;13:816025. doi: 10.3389/fphar.2022.816025. eCollection 2022. Front Pharmacol. 2022. PMID: 35281899 Free PMC article. Review.
-
A review of the botany, phytochemistry, pharmacology, synthetic biology and comprehensive utilization of Silybum marianum.Front Pharmacol. 2024 Jul 11;15:1417655. doi: 10.3389/fphar.2024.1417655. eCollection 2024. Front Pharmacol. 2024. PMID: 39055491 Free PMC article. Review.
-
Using Medicinal Plants in Valmalenco (Italian Alps): From Tradition to Scientific Approaches.Molecules. 2020 Sep 10;25(18):4144. doi: 10.3390/molecules25184144. Molecules. 2020. PMID: 32927742 Free PMC article.
References
-
- Bucala R., Cerami A. Advanced glycosylation chemistry, biology and implications for diabetes and aging. Adv. Pharmacol. 1992;23:1–4. - PubMed
-
- Hayachi C.M., Nagai R., Miyazaki K., Hayase F., Araki T., Ono T., Horiuchi S. Conversion of Amadori product of the mailard reaction to Nϵ -(carboxymethyl) lysine by short-term heating: possible detection of artefacts by immunohistochemistry. Lab. Investig. 2002;82:795–808. doi: 10.1097/01.LAB.0000018826.59648.07. - DOI - PubMed
-
- Kueper T., Grune T., Prahl S., Lenz H., Welge V., Biernoth T., Vogt Y., Muhr G.M., Gaemlich A., Jung T., et al. Vimentin is the specific target in skin glycation. Structural prerequisites, functional consequences, and role in skin aging. J. Biol. Chem. 2007;282:23427–23436. doi: 10.1074/jbc.M701586200. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

