Amine oxidative N-dealkylation via cupric hydroperoxide Cu-OOH homolytic cleavage followed by site-specific fenton chemistry
- PMID: 25706825
- PMCID: PMC4482616
- DOI: 10.1021/ja508371q
Amine oxidative N-dealkylation via cupric hydroperoxide Cu-OOH homolytic cleavage followed by site-specific fenton chemistry
Abstract
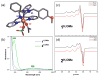
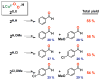
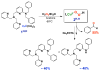
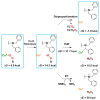
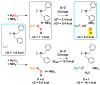
Copper(II) hydroperoxide species are significant intermediates in processes such as fuel cells and (bio)chemical oxidations, all involving stepwise reduction of molecular oxygen. We previously reported a Cu(II)-OOH species that performs oxidative N-dealkylation on a dibenzylamino group that is appended to the 6-position of a pyridyl donor of a tripodal tetradentate ligand. To obtain insights into the mechanism of this process, reaction kinetics and products were determined employing ligand substrates with various para-substituent dibenzyl pairs (-H,-H; -H,-Cl; -H,-OMe, and -Cl,-OMe), or with partially or fully deuterated dibenzyl N-(CH2Ph)2 moieties. A series of ligand-copper(II) bis-perchlorate complexes were synthesized, characterized, and the X-ray structures of the -H,-OMe analogue were determined. The corresponding metastable Cu(II)-OOH species were generated by addition of H2O2/base in acetone at -90 °C. These convert (t1/2 ≈ 53 s) to oxidatively N-dealkylated products, producing para-substituted benzaldehydes. Based on the experimental observations and supporting DFT calculations, a reaction mechanism involving dibenzylamine H-atom abstraction or electron-transfer oxidation by the Cu(II)-OOH entity could be ruled out. It is concluded that the chemistry proceeds by rate limiting Cu-O homolytic cleavage of the Cu(II)-(OOH) species, followed by site-specific copper Fenton chemistry. As a process of broad interest in copper as well as iron oxidative (bio)chemistries, a detailed computational analysis was performed, indicating that a Cu(I)OOH species undergoes O-O homolytic cleavage to yield a hydroxyl radical and Cu(II)OH rather than heterolytic cleavage to yield water and a Cu(II)-O(•-) species.
Figures





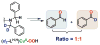

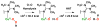

References
-
- Itoh S. Curr Opin Chem Biol. 2006;10:115–122. - PubMed
- Mirica LM, Ottenwaelder X, Stack TDP. Chem Rev. 2004;104:1013–1045. - PubMed
- Peterson RL, Kim S, Karlin KD. Comprehensive Inorganic Chemistry II. 2. Oxford: Elsevier; Amsterdam: 2013. pp. 149–177. http://dx.doi.org/10.1016/B978-0-08-097774-4.00309-0. - DOI
- Peterson RL, Ginsbach JW, Cowley RE, Qayyum MF, Himes RA, Siegler MA, Moore CD, Hedman B, Hodgson KO, Fukuzumi S, Solomon EI, Karlin KD. J Am Chem Soc. 2013;135:16454–16467. - PMC - PubMed
-
- Klinman JP. Chem Rev. 1996;96:2541–2561. - PubMed
- Klinman JP. J Biol Chem. 2006;281:3013–3016. - PubMed
- Prigge ST, Eipper B, Mains R, Amzel LM. Science. 2004;304:864–867. - PubMed
- Solomon EI, Heppner DE, Johnston EM, Ginsbach JW, Cirera J, Qayyum M, Kieber-Emmons MT, Kjaergaard CH, Hadt RG, Tian L. Chem Rev. 2014;114:3659–3853. - PMC - PubMed
-
- Chen P, Bell J, Eipper BA, Solomon EI. Biochemistry. 2004;43:5735–5747. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

