Natural photoreceptors as a source of fluorescent proteins, biosensors, and optogenetic tools
- PMID: 25706899
- PMCID: PMC4681293
- DOI: 10.1146/annurev-biochem-060614-034411
Natural photoreceptors as a source of fluorescent proteins, biosensors, and optogenetic tools
Abstract
Genetically encoded optical tools have revolutionized modern biology by allowing detection and control of biological processes with exceptional spatiotemporal precision and sensitivity. Natural photoreceptors provide researchers with a vast source of molecular templates for engineering of fluorescent proteins, biosensors, and optogenetic tools. Here, we give a brief overview of natural photoreceptors and their mechanisms of action. We then discuss fluorescent proteins and biosensors developed from light-oxygen-voltage-sensing (LOV) domains and phytochromes, as well as their properties and applications. These fluorescent tools possess unique characteristics not achievable with green fluorescent protein-like probes, including near-infrared fluorescence, independence of oxygen, small size, and photosensitizer activity. We next provide an overview of available optogenetic tools of various origins, such as LOV and BLUF (blue-light-utilizing flavin adenine dinucleotide) domains, cryptochromes, and phytochromes, enabling control of versatile cellular processes. We analyze the principles of their function and practical requirements for use. We focus mainly on optical tools with demonstrated use beyond bacteria, with a specific emphasis on their applications in mammalian cells.
Keywords: BphP; CRY2; LOV domain; bacteriophytochrome; iRFP; optogenetics; phytochrome.
Figures


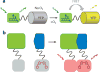


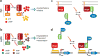
References
-
- van der Horst MA, Hellingwerf KJ. Photoreceptor proteins, “star actors of modern times”: a review of the functional dynamics in the structure of representative members of six different photoreceptor families. Acc Chem Res. 2004;37:13–20. - PubMed
-
- Christie JM, Gawthorne J, Young G, Fraser NJ, Roe AJ. LOV to BLUF: flavoprotein contributions to the optogenetic toolkit. Mol Plant. 2012;5:533–44. - PubMed
-
- Losi A, Gärtner W. The evolution of flavin-binding photoreceptors: an ancient chromophore serving trendy blue-light sensors. Annu Rev Plant Biol. 2012;63:49–72. - PubMed
-
- Chaves I, Pokorny R, Byrdin M, Hoang N, Ritz T, et al. The cryptochromes: blue light photoreceptors in plants and animals. Annu Rev Plant Biol. 2011;62:335–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

