Pluripotency gene expression and growth control in cultures of peripheral blood monocytes during their conversion into programmable cells of monocytic origin (PCMO): evidence for a regulatory role of autocrine activin and TGF-β
- PMID: 25707005
- PMCID: PMC4338298
- DOI: 10.1371/journal.pone.0118097
Pluripotency gene expression and growth control in cultures of peripheral blood monocytes during their conversion into programmable cells of monocytic origin (PCMO): evidence for a regulatory role of autocrine activin and TGF-β
Abstract
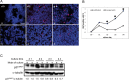
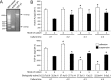
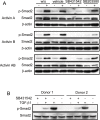
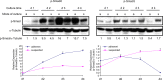
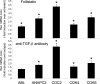
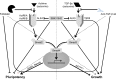
Previous studies have shown that peripheral blood monocytes can be converted in vitro to a stem cell-like cell termed PCMO as evidenced by the re-expression of pluripotency-associated genes, transient proliferation, and the ability to adopt the phenotype of hepatocytes and insulin-producing cells upon tissue-specific differentiation. However, the regulatory interactions between cultured cells governing pluripotency and mitotic activity have remained elusive. Here we asked whether activin(s) and TGF-β(s), are involved in PCMO generation. De novo proliferation of PCMO was higher under adherent vs. suspended culture conditions as revealed by the appearance of a subset of Ki67-positive monocytes and correlated with down-regulation of p21WAF1 beyond day 2 of culture. Realtime-PCR analysis showed that PCMO express ActRIIA, ALK4, TβRII, ALK5 as well as TGF-β1 and the βA subunit of activin. Interestingly, expression of ActRIIA and ALK4, and activin A levels in the culture supernatants increased until day 4 of culture, while levels of total and active TGF-β1 strongly declined. PCMO responded to both growth factors in an autocrine fashion with intracellular signaling as evidenced by a rise in the levels of phospho-Smad2 and a drop in those of phospho-Smad3. Stimulation of PCMO with recombinant activins (A, B, AB) and TGF-β1 induced phosphorylation of Smad2 but not Smad3. Inhibition of autocrine activin signaling by either SB431542 or follistatin reduced both Smad2 activation and Oct4A/Nanog upregulation. Inhibition of autocrine TGF-β signaling by either SB431542 or anti-TGF-β antibody reduced Smad3 activation and strongly increased the number of Ki67-positive cells. Furthermore, anti-TGF-β antibody moderately enhanced Oct4A/Nanog expression. Our data show that during PCMO generation pluripotency marker expression is controlled positively by activin/Smad2 and negatively by TGF-β/Smad3 signaling, while relief from growth inhibition is primarily the result of reduced TGF-β/Smad3, and to a lesser extent, activin/Smad2 signaling.
Conflict of interest statement
Figures

Similar articles
-
Transforming growth factor-beta- and Activin-Smad signaling pathways are activated at distinct maturation stages of the thymopoeisis.Int Immunol. 2003 Dec;15(12):1401-14. doi: 10.1093/intimm/dxg139. Int Immunol. 2003. PMID: 14645149
-
Role of ALK5/Smad2/3 and MEK1/ERK Signaling in Transforming Growth Factor Beta 1-modulated Growth, Collagen Turnover, and Differentiation of Stem Cells from Apical Papilla of Human Tooth.J Endod. 2015 Aug;41(8):1272-80. doi: 10.1016/j.joen.2015.03.022. Epub 2015 May 19. J Endod. 2015. PMID: 26001858
-
Activin A, B and AB decrease progesterone production by down-regulating StAR in human granulosa cells.Mol Cell Endocrinol. 2015 Sep 5;412:290-301. doi: 10.1016/j.mce.2015.05.016. Epub 2015 May 19. Mol Cell Endocrinol. 2015. PMID: 26001835
-
Intracrine signaling mechanisms of activin A and TGF-β.Vitam Horm. 2011;85:59-77. doi: 10.1016/B978-0-12-385961-7.00004-4. Vitam Horm. 2011. PMID: 21353876 Review.
-
Growth and differentiation factor 11 (GDF11): Functions in the regulation of erythropoiesis and cardiac regeneration.Pharmacol Ther. 2015 Dec;156:26-33. doi: 10.1016/j.pharmthera.2015.10.006. Epub 2015 Oct 30. Pharmacol Ther. 2015. PMID: 26523637 Review.
Cited by
-
Transfection of Peripheral Blood Monocytes with SOX2 Enhances Multipotency, Proliferation, and Redifferentiation into Neohepatocytes and Insulin-Producing Cells.Stem Cells Int. 2018 Oct 4;2018:4271875. doi: 10.1155/2018/4271875. eCollection 2018. Stem Cells Int. 2018. PMID: 30402109 Free PMC article.
-
Peripheral Blood Monocytes as Adult Stem Cells: Molecular Characterization and Improvements in Culture Conditions to Enhance Stem Cell Features and Proliferative Potential.Stem Cells Int. 2016;2016:7132751. doi: 10.1155/2016/7132751. Epub 2015 Dec 20. Stem Cells Int. 2016. PMID: 26798361 Free PMC article. Review.
-
Mesangiogenic progenitor cells: a mesengenic and vasculogenic branch of hemopoiesis? A story of neglected plasticity.Front Cell Dev Biol. 2025 Mar 24;13:1513440. doi: 10.3389/fcell.2025.1513440. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40196849 Free PMC article.
-
Inhibitory Antibodies against Activin A and TGF-β Reduce Self-Supported, but Not Soluble Factors-Induced Growth of Human Pulmonary Arterial Vascular Smooth Muscle Cells in Pulmonary Arterial Hypertension.Int J Mol Sci. 2018 Sep 28;19(10):2957. doi: 10.3390/ijms19102957. Int J Mol Sci. 2018. PMID: 30274147 Free PMC article.
References
-
- Ruhnke M, Ungefroren H, Nussler A, Martin F, Brulport M, et al. (2005) Differentiation of in vitro-modified human peripheral blood monocytes into hepatocyte-like and pancreatic islet- like cells. Gastroenterology 128: 1774–1786. - PubMed
-
- Ruhnke M, Nussler AK, Ungefroren H, Hengstler JG, Kremer B, et al. (2005) Human monocyte-derived neohepatocytes: a promising alternative to primary human hepatocytes for autologous cell therapy. Transplantation 79: 1097–1103. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

