Circadian regulation of cellular physiology
- PMID: 25707277
- PMCID: PMC4690540
- DOI: 10.1016/bs.mie.2014.10.006
Circadian regulation of cellular physiology
Abstract
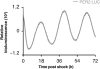
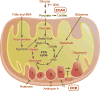
The circadian clock synchronizes behavioral and physiological processes on a daily basis in anticipation of the light-dark cycle. In mammals, molecular clocks are present in both the central pacemaker neurons and in nearly all peripheral tissues. Clock transcription factors in metabolic tissues coordinate metabolic fuel utilization and storage with alternating periods of feeding and fasting corresponding to the rest-activity cycle. In vitro and in vivo biochemical approaches have led to the discovery of mechanisms underlying the interplay between the molecular clock and the metabolic networks. For example, recent studies have demonstrated that the circadian clock controls rhythmic synthesis of the cofactor nicotinamide adenine dinucleotide (NAD(+)) and activity of NAD(+)-dependent sirtuin deacetylase enzymes to regulate mitochondrial function across the circadian cycle. In this chapter, we review current state-of-the-art methods to analyze circadian cycles in mitochondrial bioenergetics, glycolysis, and nucleotide metabolism in both cell-based and animal models.
Keywords: Circadian; Energetics; Metabolism; Mitochondria; NAD; Respiration; Sirtuin.
© 2015 Elsevier Inc. All rights reserved.
Figures


References
-
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. - PubMed
-
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. - PubMed
-
- Balsalobre A, Marcacci L, Schibler U. Multiple signaling pathways elicit circadian gene expression in cultured Rat-1 fibroblasts. Current Biology. 2000;10:1291–1294. - PubMed
-
- Bennett MJ. Assays of fatty acid beta-oxidation activity. Methods in Cell Biology. 2007;80:179–197. - PubMed
-
- Boden G, Ruiz J, Urbain JL, Chen X. Evidence for a circadian rhythm of insulin secretion. The American Journal of Physiology. 1996;271:E246–E252. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

