Acetyl-lysine erasers and readers in the control of pulmonary hypertension and right ventricular hypertrophy
- PMID: 25707943
- PMCID: PMC4975937
- DOI: 10.1139/bcb-2014-0119
Acetyl-lysine erasers and readers in the control of pulmonary hypertension and right ventricular hypertrophy
Abstract
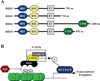
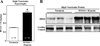
Acetylation of lysine residues within nucleosomal histone tails provides a crucial mechanism for epigenetic control of gene expression. Acetyl groups are coupled to lysine residues by histone acetyltransferases (HATs) and removed by histone deacetylases (HDACs), which are also commonly referred to as "writers" and "erasers", respectively. In addition to altering the electrostatic properties of histones, lysine acetylation often creates docking sites for bromodomain-containing "reader" proteins. This review focuses on epigenetic control of pulmonary hypertension (PH) and associated right ventricular (RV) cardiac hypertrophy and failure. Effects of small molecule HDAC inhibitors in pre-clinical models of PH are highlighted. Furthermore, we describe the recently discovered role of bromodomain and extraterminal (BET) reader proteins in the control of cardiac hypertrophy, and provide evidence suggesting that one member of this family, BRD4, contributes to the pathogenesis of RV failure. Together, the data suggest intriguing potential for pharmacological epigenetic therapies for the treatment of PH and right-sided heart failure.
L'acétylation des lysines situées á l'intérieur des queues des histones nucléosomales constitue un mécanisme crucial de contrôle épigénétique de l'expression génique. Les groupes acétyles sont couplés aux lysines par les histone acétytransférases (HAT) et sont enlevés par les histone déacétylases (HDAC), dont on dit qu'elles sont respectivement des « rédacteurs » et des « effaceurs ». En plus de modifier les propriétés électrostatiques des histones, l'acétylation des lysines crée souvent un site d'amarrage des protéines comportant un bromodomaine qu'on dit « lectrices ». Cet article de revue se concentre sur le contrôle épigénétique de l‘hypertension pulmonaire (HP), et de l'hypertrophie et de l'insuffisance cardiaques ventriculaires droites qui lui sont associées. Les effets de petites molécules qui inhibent les HDAC dans des modèles précliniques d'HP sont soulignés. De plus, les auteurs décrivent le rôle récemment découvert des protéines lectrices BET (bromodomaine et domaine terminal) dans le contrôle de l'hypertrophie cardiaque, et présentent des indices qui suggèrent qu'un membre de cette famille, BRD4, contribue á la pathogenèse de l'insuffisance ventriculaire droite. Dans l'ensemble, les données suggèrent que les thérapies pharmacologiques épigénétiques possèdent un potentiel de traitement intéressant de l'HP et de l'insuffisance cardiaque localisée du côté droit. [Traduit par la Rédaction]
Keywords: HDAC; RV hypertrophy; bromodomain; bromodomaine; epigenetics; hypertension pulmonaire; hypertrophie ventriculaire droite; pulmonary hypertension; épigénétique.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

