CD47 signaling pathways controlling cellular differentiation and responses to stress
- PMID: 25708195
- PMCID: PMC4822708
- DOI: 10.3109/10409238.2015.1014024
CD47 signaling pathways controlling cellular differentiation and responses to stress
Abstract
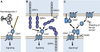
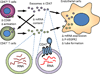
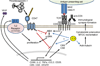
CD47 is a widely expressed integral membrane protein that serves as the counter-receptor for the inhibitory phagocyte receptor signal-regulatory protein-α (SIRPα) and as a signaling receptor for the secreted matricellular protein thrombospondin-1. Recent studies employing mice and somatic cells lacking CD47 have revealed important pathophysiological functions of CD47 in cardiovascular homeostasis, immune regulation, resistance of cells and tissues to stress and chronic diseases of aging including cancer. With the emergence of experimental therapeutics targeting CD47, a more thorough understanding of CD47 signal transduction is essential. CD47 lacks a substantial cytoplasmic signaling domain, but several cytoplasmic binding partners have been identified, and lateral interactions of CD47 with other membrane receptors play important roles in mediating signaling resulting from the binding of thrombospondin-1. This review addresses recent advances in identifying the lateral binding partners, signal transduction pathways and downstream transcription networks regulated through CD47 in specific cell lineages. Major pathways regulated by CD47 signaling include calcium homeostasis, cyclic nucleotide signaling, nitric oxide and hydrogen sulfide biosynthesis and signaling and stem cell transcription factors. These pathways and other undefined proximal mediators of CD47 signaling regulate cell death and protective autophagy responses, mitochondrial biogenesis, cell adhesion and motility and stem cell self-renewal. Although thrombospondin-1 is the best characterized agonist of CD47, the potential roles of other members of the thrombospondin family, SIRPα and SIRPγ binding and homotypic CD47 interactions as agonists or antagonists of signaling through CD47 should also be considered.
Keywords: Autophagy; Myc; hydrogen sulfide; immune regulation; integrin signaling; nitric oxide; radioresistance; stem cells.
Figures






References
-
- Adams JC, Bentley AA, Kvansakul M, Hatherley D, Hohenester E. Extracellular matrix retention of thrombospondin 1 is controlled by its conserved C-terminal region. J Cell Sci. 2008;121:784–795. - PubMed
-
- Avent ND, Madgett TE, Lee ZE, Head DJ, Maddocks DG, Skinner LH. Molecular biology of Rh proteins and relevance to molecular medicine. Expert Rev Mol Med. 2006;8:1–20. - PubMed
-
- Azcutia V, Routledge M, Williams MR, Newton G, Frazier WA, Manica A, Croce KJ, Parkos CA, Schmider AB, Turman MV, Soberman RJ, Luscinskas FW. CD47 plays a critical role in T-cell recruitment by regulation of LFA-1 and VLA-4 integrin adhesive functions. Mol Biol Cell. 2013;24:3358–3368. - PMC - PubMed
-
- Barazi HO, Li Z, Cashel JA, Krutzsch HC, Annis DS, Mosher DF, Roberts DD. Regulation of integrin function by CD47 ligands. Differential effects on avb3 and a4b1 integrin-mediated adhesion. J Biol Chem. 2002;277:42859–42866. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
