Experimental characterization of adsorbed protein orientation, conformation, and bioactivity
- PMID: 25708632
- PMCID: PMC4312349
- DOI: 10.1116/1.4906485
Experimental characterization of adsorbed protein orientation, conformation, and bioactivity
Abstract
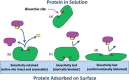
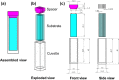
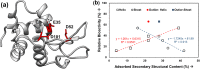
Protein adsorption on material surfaces is a common phenomenon that is of critical importance in many biotechnological applications. The structure and function of adsorbed proteins are tightly interrelated and play a key role in the communication and interaction of the adsorbed proteins with the surrounding environment. Because the bioactive state of a protein on a surface is a function of the orientation, conformation, and accessibility of its bioactive site(s), the isolated determination of just one or two of these factors will typically not be sufficient to understand the structure-function relationships of the adsorbed layer. Rather a combination of methods is needed to address each of these factors in a synergistic manner to provide a complementary dataset to characterize and understand the bioactive state of adsorbed protein. Over the past several years, the authors have focused on the development of such a set of complementary methods to address this need. These methods include adsorbed-state circular dichroism spectropolarimetry to determine adsorption-induced changes in protein secondary structure, amino-acid labeling/mass spectrometry to assess adsorbed protein orientation and tertiary structure by monitoring adsorption-induced changes in residue solvent accessibility, and bioactivity assays to assess adsorption-induced changes in protein bioactivity. In this paper, the authors describe the methods that they have developed and/or adapted for each of these assays. The authors then provide an example of their application to characterize how adsorption-induced changes in protein structure influence the enzymatic activity of hen egg-white lysozyme on fused silica glass, high density polyethylene, and poly(methyl-methacrylate) as a set of model systems.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

