Paraoxonase-2 (PON2) protects oral squamous cell cancer cells against irradiation-induced apoptosis
- PMID: 25708945
- PMCID: PMC11823691
- DOI: 10.1007/s00432-015-1941-2
Paraoxonase-2 (PON2) protects oral squamous cell cancer cells against irradiation-induced apoptosis
Abstract
Purpose: Patients with oral squamous cell carcinomas (OSCC) often receive radiotherapy to preferentially induce apoptosis of cancer cells through generation of overwhelming DNA damage. This is amplified by generation of reactive oxygen species (ROS), thereby causing oxidative stress and cell death. However, tumors resist through different mechanisms, including upregulation of anti-apoptotic factors and enhanced ROS resistance. We recently reported that the antioxidative enzyme PON2 significantly enhances cellular stress resistance by attenuating mitochondrial ROS-mediated apoptosis. Further, PON2 is often upregulated in cancer. This prompted us to investigate its yet unknown role in the protection of OSCC against irradiation-induced cell death.
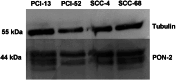
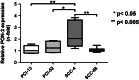
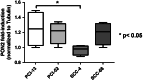
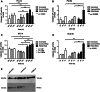
Methods: PON2 expression was determined after 7 Gy singular irradiation in four OSCC cell lines (PCI-13, PCI-52, SCC-4, SCC-68) accompanied by the detection of caspase 3/7 activity. A direct role of PON2 was tested by siRNA-mediated knockdown. In vivo PON2 expression was tested in five patients with oral carcinoma and compared with healthy mucosa for the evaluation of clinical significance.
Results: PON2 is variably expressed in OSCC in vitro and in vivo. Compared with the other cell lines, SCC-4 cells showed twofold more basal PON2 (p ≤ 0.05) and the lowest caspase 3/7 activity after singular irradiation (p ≤ 0.05). Contrarily, irradiation led to 1.2-fold induction of PON2 in PCI-13 with no effect on SCC-4 (≤0.05), suggesting that PON2 levels reflect the cells' irradiation sensitivity. In agreement, PON2 knockdown resulted in significant higher apoptosis rates (p ≤ 0.05).
Conclusion: Our findings give first evidence that upregulation of PON2 may protect OSCC against irradiation-induced apoptosis.
Conflict of interest statement
The authors declare that they have no conflict of interest. All authors read and approved the final manuscript.
Figures


References
-
- Benhar M, Dalyot I, Engelberg D, Levitzki A (2001) Enhanced ROS production in oncogenically transformed cells potentiates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase activation and sensitization to genotoxic stress. Mol Cell Biol 21:6913–6926. doi:10.1128/MCB.21.20.6913-6926.2001 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

