Efficacy and safety of a herbal mixture (Viron® tablets) in the treatment of patients with chronic hepatitis C virus infection: a prospective, randomized, open-label, proof-of-concept study
- PMID: 25709404
- PMCID: PMC4334351
- DOI: 10.2147/DDDT.S77168
Efficacy and safety of a herbal mixture (Viron® tablets) in the treatment of patients with chronic hepatitis C virus infection: a prospective, randomized, open-label, proof-of-concept study
Abstract
Background: Development of an optimal interferon-free regimen for chronic hepatitis C virus infection is believed to require the combination of different drug classes to provide good antiviral efficacy, clinical and quality of life benefits, as well as a high barrier to resistance. Viron(®) is a new herbal drug in film-coated tablet form, and is based on a mixture of herbs with known hepatoprotective and antiviral properties. We conducted this study to explore the safety and the potential clinical and quality of life benefits of this product in patients with chronic hepatitis C infection.
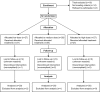
Methods: Eighty-two consecutive patients presenting to our outpatient clinics as already-known or newly-diagnosed cases of chronic hepatitis C virus (HCV) infection, were entered into the study and randomized to three groups to receive escalating doses of Viron for 6 months. Virological, clinical, and enzyme responses, as well as quality of life index scores for chronic liver disease were compared between the groups.
Results: Of the 20 patients treated with the highest dose of Viron (three tablets twice daily), two (10%) had a complete virological response at the end of treatment (ETR) and two (10%) had a partial ETR, defined as a decrease in viral load of at least 2-log10 at the end of 6 months of treatment, whereas patients treated with the medium dose (two tablets twice daily) and the lowest dose (one tablet twice daily) showed a significantly lower ETR (P=0.043). Alanine aminotransferase levels and scores on the Chronic Liver Disease Questionnaire improved to a significantly greater extent in the highest dose group (P=0.007 and P=0.021, respectively). No serious adverse effects attributable to the herbal formulation were reported in any of the groups, apart from mild transient nausea, bloating, giddiness, and headache in two patients in the group receiving two tablets twice daily and in three patients in the group receiving three tablets twice daily.
Conclusion: We conclude that this herbal formulation is potentially safe and may offer some added clinical and quality of life benefits when used in the treatment of patients with chronic hepatitis C virus infection. Larger studies could be warranted to evaluate the effects of using this formulation as an add-on therapy to an all-oral combination of a directly acting antiviral drug protocol in the treatment of chronic hepatitis C.
Keywords: chronic hepatitis C virus; efficacy; proof-of-concept study; safety.
Similar articles
-
Telaprevir twice daily is noninferior to telaprevir every 8 hours for patients with chronic hepatitis C.Gastroenterology. 2014 Mar;146(3):744-753.e3. doi: 10.1053/j.gastro.2013.11.047. Epub 2013 Dec 4. Gastroenterology. 2014. PMID: 24316262 Clinical Trial.
-
Efficacy of an interferon- and ribavirin-free regimen of daclatasvir, asunaprevir, and BMS-791325 in treatment-naive patients with HCV genotype 1 infection.Gastroenterology. 2014 Feb;146(2):420-9. doi: 10.1053/j.gastro.2013.10.057. Epub 2013 Oct 30. Gastroenterology. 2014. PMID: 24184132 Clinical Trial.
-
Sofosbuvir plus ribavirin for treatment of hepatitis C virus in patients co-infected with HIV (PHOTON-2): a multicentre, open-label, non-randomised, phase 3 study.Lancet. 2015 Mar 21;385(9973):1098-106. doi: 10.1016/S0140-6736(14)62483-1. Epub 2015 Feb 4. Lancet. 2015. PMID: 25659285 Clinical Trial.
-
Hepatitis C virus infection: quality of life and side effects of treatment.J Hepatol. 1999;31 Suppl 1:250-4. doi: 10.1016/s0168-8278(99)80411-5. J Hepatol. 1999. PMID: 10622597 Review.
-
[Chronic hepatitis by C virus. Part 2. Treatment].Arq Gastroenterol. 2000 Oct-Dec;37(4):235-42. doi: 10.1590/s0004-28032000000400010. Arq Gastroenterol. 2000. PMID: 11460605 Review. Portuguese.
Cited by
-
The very-rapid and the ultra-rapid virologic response to two treatment options in patients with chronic hepatitis C: an interim report of a prospective randomized comparative effectiveness study.Drug Des Devel Ther. 2015 Nov 11;9:6027-33. doi: 10.2147/DDDT.S95499. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26628861 Free PMC article. Clinical Trial.
References
-
- World Health Organization Hepatitis C. Fact sheet 164. [Accessed July 3, 2014]. Available from: http://www.who.int/mediacentre/factsheets/fs164/en/
-
- Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47:1–39. [No authors listed] - PubMed
-
- Sievert W, Altraif I, Razavi HA, et al. systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int. 2011;31:61–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources