Clinical pharmacology of paracetamol in neonates: a review
- PMID: 25709719
- PMCID: PMC4329422
- DOI: 10.1016/j.curtheres.2014.12.001
Clinical pharmacology of paracetamol in neonates: a review
Abstract
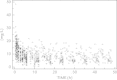
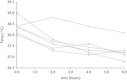
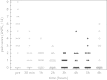
Paracetamol is commonly used to control mild-to-moderate pain or to reduce opioid exposure as part of multimodal analgesia, and is the only compound recommended to treat fever in neonates. Paracetamol clearance is lower in neonates than in children and adults. After metabolic conversion, paracetamol is subsequently eliminated by the renal route. The main metabolic conversions are conjugation with glucuronic acid and with sulphate. In the urine of neonates sulphated paracetamol concentration is higher than the glucuronidated paracetamol level, suggesting that sulfation prevails over glucuronidation in neonates. A loading dose of 20 mg/kg followed by 10 mg/kg every 6 hours of intravenous paracetamol is suggested to achieve a compartment concentration of 11 mg/L in late preterm and term neonates. Aiming for the same target concentration, oral doses are similar with rectal administration of 25 to 30 mg/kg/d in preterm neonates of 30 weeks' gestation, 45 mg/kg/d in preterm infants of 34 weeks' gestation, and 60 mg/kg/d in term neonates are suggested. The above-mentioned paracetamol doses for these indications (pain, fever) are well tolerated in neonates, but do not result in a significant increase in liver enzymes, and do not affect blood pressure and have limited effects on heart rate. In contrast, the higher doses suggested in extreme preterm neonates to induce closure of the patent ductus arteriosus have not yet been sufficiently evaluated regarding efficacy or safety. Moreover, focussed pharmacovigilance to explore the potential causal association between paracetamol exposure during perinatal life and infancy and subsequent atopy is warranted.
Keywords: Dosing; glucuronidation; metabolism; neonate; paracetamol; sulfation.
Figures
References
-
- Prescott L.F. A critical bibliographic review. Second edition. Taylor and Francis publishers; London: 2001. Paracetamol.
-
- Cuzzolin L., Antonucci R., Fanos V. Paracetamol (acetaminophen) efficacy and safety in the newborn. Curr Drug Metab. 2013;14:178–185. - PubMed
-
- Duggan S.T., Scott L.J. Intravenous paracetamol (acetaminophen) Drugs. 2009;69:101–113. - PubMed
-
- Miller R.P., Roberts R.J., Fisher L.J. Acetaminophen elimination kinetics in neonates, children and adults. Clin Pharmacol Ther. 1976;19:284–294. - PubMed
-
- Anderson B.J., van Lingen R.A., Hansen T.G., Lin Y.C., Holford N.H. Acetaminophen developmental pharmacokinetics in premature neonates and infants: a pooled population analysis. Anesthesiology. 2002;96:1336–1345. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

