Bevacizumab versus ranibizumab for neovascular age-related macular degeneration: a Meta-analysis
- PMID: 25709924
- PMCID: PMC4325258
- DOI: 10.3980/j.issn.2222-3959.2015.01.26
Bevacizumab versus ranibizumab for neovascular age-related macular degeneration: a Meta-analysis
Abstract
Aim: To systematically compare the efficacy and safety of off-label bevacizumab versus licensed ranibizumab intravitreal injections as well as monthly regimen versus pro re nata [PRN (as needed)] regimen in the treatment of neovascular age-related macular degeneration (nAMD).
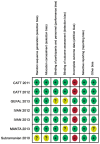
Methods: Relevant publications were identified through automatically retrieve of database and manually retrieving. The methodological quality of studies included was assessed using the Jadad score and the risk-of-bias assessment. The efficacy estimates were measured by the weight mean difference (WMD) for the improvement of best-corrected visual acuity (BCVA) and central retinal thickness (CRT) reduction. The safety estimates were measured by odds ratios (OR) for adverse events rates. Statistical analysis was conducted by Revman 5.2.7.
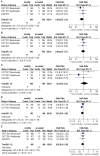
Results: Seven studies were included in the Meta-analysis. There were no statistically significant differences between bevacizumab and ranibizumab in BCVA at 1 and 2y (P=0.37, P=0.18, respectively), However, both drugs has better BCVA given monthly than given as needed at 1 and 2y (P<0.05). The results demonstrated the mean decrease in CRT was less in bevacizumab group than ranibizumab group at 1y (P<0.05), while the difference was not significant at 2y (P=0.24). Treatment monthly gained much more decrease in CRT at 1 and 2y (P<0.005). There were no differences between drugs in the rates of death, arterial thrombotic events and venous thrombotic events (P=0.41, P=0.55, P=0.10, respectively), while the rates of medical dictionary for regulatory activities (MedDAR) system organ class events and ≥1 systemic serious adverse events were higher in bevacizumab group than ranibizumab group (P<0.05). But the incidences of death, arterial thrombotic events, venous thrombotic events, MedDAR system organ class events as well as ≥1 systemic serious adverse events were not statistically different between both treatment regimens of monthly and as needed (P=0.14, P=0.76, P=0.73, P=0.12, P=0.11, respectively).
Conclusion: Bevacizumab was equivalent to ranibizumab for BCVA, however bevacizumab tended to gain less decrease in CRT and had higher rates of serious adverse events. Compared with treatment as needed, treatment monthly showed superior efficacy in BCVA improvement and CRT reduction, while the rates of adverse events were similar in the two dosing regimens.
Keywords: Meta-analysis; bevacizumab; neovascular age-related macular degeneration; ranibizumab.
Figures





References
-
- Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA. 2004;291(15):1900–1901. - PubMed
-
- Takahashi K, Ishibashi T, Ogura Y, Yuzawa M, Working Group for Establishing Diagnostic Criteria for Age-Related Macular Degeneration Classification and diagnostic criteria of age-related macular degeneration. Nihon Ganka Gakkai Zasshi. 2008;112(12):1076–1084. - PubMed
-
- Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438(7070):967–974. - PubMed
-
- Kvanta A, Algvere PV, Berglin L, Seregard S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 1996;37(9):1929–1934. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
