Gene expression in obliterative bronchiolitis-like lesions in 2,3-pentanedione-exposed rats
- PMID: 25710175
- PMCID: PMC4339611
- DOI: 10.1371/journal.pone.0118459
Gene expression in obliterative bronchiolitis-like lesions in 2,3-pentanedione-exposed rats
Abstract
Obliterative bronchiolitis (OB) is an irreversible lung disease characterized by progressive fibrosis in the small airways with eventual occlusion of the airway lumens. OB is most commonly associated with lung transplant rejection; however, OB has also been diagnosed in workers exposed to artificial butter flavoring (ABF) vapors. Research has been limited by the lack of an adequate animal model of OB, and as a result the mechanism(s) is unclear and there are no effective treatments for this condition. Exposure of rats to the ABF component, 2,3-pentanedione (PD) results in airway lesions that are histopathologically similar to those in human OB. We used this animal model to evaluate changes in gene expression in the distal bronchi of rats with PD-induced OB. Male Wistar Han rats were exposed to 200 ppm PD or air 6 h/d, 5 d/wk for 2-wks. Bronchial tissues were laser microdissected from serial sections of frozen lung. In exposed lungs, both fibrotic and non-fibrotic airways were collected. Following RNA extraction and microarray analysis, differential gene expression was evaluated. In non-fibrotic bronchi of exposed rats, 4683 genes were significantly altered relative to air-exposed controls with notable down-regulation of many inflammatory cytokines and chemokines. In contrast, in fibrotic bronchi, 3807 genes were significantly altered with a majority of genes being up-regulated in affected pathways. Tgf-β2 and downstream genes implicated in fibrosis were significantly up-regulated in fibrotic lesions. Genes for collagens and extracellular matrix proteins were highly up-regulated. In addition, expression of genes for peptidases and peptidase inhibitors were significantly altered, indicative of the tissue remodeling that occurs during airway fibrosis. Our data provide new insights into the molecular mechanisms of OB. This new information is of potential significance with regard to future therapeutic targets for treatment.
Conflict of interest statement
Figures

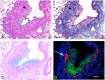
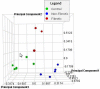

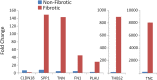
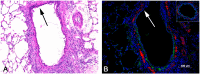
References
-
- Ryu JH, Myers J, Swenson SJ. Bronchiolar disorders. Am J Resp Crit Care Med. 2003;168(11):1277–92. - PubMed
-
- Kreiss K, Gomaa A, Kullman G, Fedan K, Simoes EJ, Enright PL. Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. N Engl J Med. 2002;347(5):330–8. . - PubMed
-
- Morgan DL, Jokinen MP, Johnson CL, Gwinn WM, Price HC, Flake GP. Bronchial fibrosis in rats exposed to 2,3-butanedione and 2,3-pentanedione. Toxicologist. 2012;126(1):866.
-
- Weng L, Dai H, Zhan Y, He Y, Stepaniants S, Bassett DE. Rosetta error model for gene expression analysis. Bioinformatics. 2006;22(9):1111–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

