Practical synthesis of cytidine-5-carboxamide-modified nucleotide reagents
- PMID: 25710355
- PMCID: PMC4353258
- DOI: 10.1080/15257770.2014.978011
Practical synthesis of cytidine-5-carboxamide-modified nucleotide reagents
Abstract
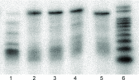
Chemically-modified derivatives of cytidine, bearing a 5-(N-substituted-carboxamide) functional group, are new reagents for use in aptamer discovery via the SELEX process (Systematic Evolution of Ligands by EXponential enrichment). Herein, we disclose a practical synthesis of 5-(N-benzylcarboxamide)-2'-deoxycytidine, and the corresponding 5-(N-1-naphthylmethylcarboxamide)- and 5-(N-3-phenylpropylcarboxamide)-2'-deoxycytidine analogs, as both the suitably-protected 3'-O-cyanoethylphosphoramidite reagents (CEP; gram scale) and the 5'-O-triphosphate reagents (TPP; milligram-scale). The key step in the syntheses is a mild, palladium(0)-catalyzed carboxyamidation of an unprotected 5-iodo-cytidine. Use of the CEP reagents for solid-phase oligonucleotide synthesis was demonstrated and incorporation of the TPP reagents by KOD polymerase in a primer extension assay confirmed the utility of these reagents for SELEX. Finally, the carboxyamidation reaction was also used to prepare the nuclease-resistant sugar-variants: 5-(N-benzylcarboxamide)-2'-O-methyl-cytidine and 5-(N-3-phenylpropylcarboxamide)-2'-deoxy-2'-fluoro-cytidine.
Keywords: Aptamer; SELEX; cytidine-5-carboxamide; modified nucleotide.
Figures

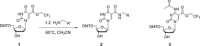

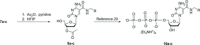

References
-
- Hollenstein M. Synthesis of deoxynucleoside triphosphates that include proline, Urea, or sulfonamide groups and their polymerase incorporation into DNA. Chem., A Eur. J. 2012;18:13320–13330. - PubMed
-
- Imaizumi Y. Kasahara Y. Fujita H. Kitadume S. Ozaki H. Endoh T. Kuwahara M. Sugimoto N. Efficacy of base-modification on target binding of small molecule DNA aptamers. J. Am. Chem. Soc. 2013;135(25):9412–9419. - PubMed
-
- Davies D.R. Gelinas A.D. Zhang C. Rohloff J.C. Carter J.D. O’Connell D. Waugh S.M. Wolk S.M. Mayfield W.S. Burgin A.B. Edwards T.E. Stewart L.J. Gold L. Janjic N. Jarvis T.C. Unique motifs and hydrophobic interactions shape the binding of modified DNA ligands to protein targets. PNAS. 2012;190(49):19971–19976. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
