Phase I/II study of the combination of panobinostat and carfilzomib in patients with relapsed/refractory multiple myeloma
- PMID: 25710456
- PMCID: PMC4420216
- DOI: 10.3324/haematol.2014.119735
Phase I/II study of the combination of panobinostat and carfilzomib in patients with relapsed/refractory multiple myeloma
Abstract
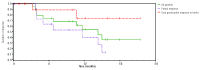
The purpose of this study was to assess the safety and efficacy of the combination of panobinostat and carfilzomib in patients with relapsed/refractory multiple myeloma. Patients with multiple myeloma who had relapsed after at least one prior treatment were eligible to participate. In the dose escalation part of the study a standard 3+3 design was used to determine the maximum tolerated dose of four planned dose levels of the combination of carfilzomib and panobinostat. Panobinostat was administered on days 1, 3, 5, 15, 17, and 19. Carfilzomib was administered on days 1, 2, 8, 9, 15, and 16 of each 28-day cycle. Treatment was continued until progression or intolerable toxicity. Forty-four patients were accrued into the trial, 13 in the phase I part and 31 in the phase II part of the study. The median age of the patients was 66 years and the median number of prior therapies was five. The expansion dose was established as 30 mg panobinostat, 20/45 mg/m(2) carfilzomib. The overall response rate was 67% for all patients, 67% for patients refractory to prior proteasome inhibitor treatment and 75% for patients refractory to prior immune modulating drug treatment. At a median follow up of 17 months, median progression-free survival was 7.7 months, median time to progression was 7.7 months, and median overall survival had not been reached. The regimen was well tolerated, although there were several panobinostat dose reductions. In conclusion, the combination of panobinostat and carfilzomib is feasible and effective in patients with relapsed/refractory multiple myeloma. (Trial registered at ClinicalTrials.gov: NCT01496118).
Copyright© Ferrata Storti Foundation.
Figures
References
-
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046–1060. - PubMed
-
- National Cancer Institute. (homepage on the internet.) Cited 2014. Available from: http://seer.cancer.gov/statfacts/html/mulmy.html.
-
- Kumar SK, Therneau TM, Gertz MA, et al. Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc. 2004;79(7):867–874. - PubMed
-
- Cavo M. Proteasome inhibitor bortezomib for the treatment of multiple myeloma. Leukemia. 2006;20(8):1341–1352. - PubMed
-
- Demo SD, Kirk CJ, Aujay MA, et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 2007;67(13):6383–6391. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical