The evolution of early neurogenesis
- PMID: 25710527
- PMCID: PMC5987553
- DOI: 10.1016/j.devcel.2015.02.004
The evolution of early neurogenesis
Abstract
The foundation of the diverse metazoan nervous systems is laid by embryonic patterning mechanisms, involving the generation and movement of neural progenitors and their progeny. Here we divide early neurogenesis into discrete elements, including origin, pattern, proliferation, and movement of neuronal progenitors, which are controlled by conserved gene cassettes. We review these neurogenetic mechanisms in representatives of the different metazoan clades, with the goal to build a conceptual framework in which one can ask specific questions, such as which of these mechanisms potentially formed part of the developmental "toolkit" of the bilaterian ancestor and which evolved later.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

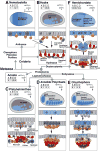

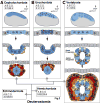


References
-
- Aguinaldo AM, Turbeville JM, Linford LS, Rivera MC, Garey JR, Raff RA, Lake JA. Evidence for a clade of nematodes, arthropods and other moulting animals. Nature. 1997;387:489–493. - PubMed
-
- Altmann CR, Brivanlou AH. Neural patterning in the vertebrate embryo. Int Rev Cytol. 2001;203:447–482. - PubMed
-
- Ashraf SI, Ip YT. The Snail protein family regulates neuroblast expression of inscuteable and string, genes involved in asymmetry and cell division in Drosophila. Development. 2001;128:4757–4767. - PubMed

