The Effects of Cinacalcet in Older and Younger Patients on Hemodialysis: The Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) Trial
- PMID: 25710802
- PMCID: PMC4422239
- DOI: 10.2215/CJN.07730814
The Effects of Cinacalcet in Older and Younger Patients on Hemodialysis: The Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) Trial
Abstract
Background and objectives: The calcimimetic cinacalcet reduced the risk of death or cardiovascular (CV) events in older, but not younger, patients with moderate to severe secondary hyperparathyroidism (HPT) who were receiving hemodialysis. To determine whether the lower risk in younger patients might be due to lower baseline CV risk and more frequent use of cointerventions that reduce parathyroid hormone (kidney transplantation, parathyroidectomy, and commercial cinacalcet use), this study examined the effects of cinacalcet in older (≥65 years, n=1005) and younger (<65 years, n=2878) patients.
Design, setting, participants, & measurements: Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) was a global, multicenter, randomized placebo-controlled trial in 3883 prevalent patients on hemodialysis, whose outcomes included death, major CV events, and development of severe unremitting HPT. The age subgroup analysis was prespecified.
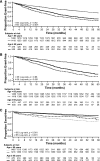
Results: Older patients had higher baseline prevalence of diabetes mellitus and CV comorbidity. Annualized rates of kidney transplantation and parathyroidectomy were >3-fold higher in younger relative to older patients and were more frequent in patients randomized to placebo. In older patients, the adjusted relative hazard (95% confidence interval) for the primary composite (CV) end point (cinacalcet versus placebo) was 0.70 (0.60 to 0.81); in younger patients, the relative hazard was 0.97 (0.86 to 1.09). Corresponding adjusted relative hazards for mortality were 0.68 (0.51 to 0.81) and 0.99 (0.86 to 1.13). Reduction in the risk of severe unremitting HPT was similar in both groups.
Conclusions: In the EVOLVE trial, cinacalcet decreased the risk of death and of major CV events in older, but not younger, patients with moderate to severe HPT who were receiving hemodialysis. Effect modification by age may be partly explained by differences in underlying CV risk and differential application of cointerventions that reduce parathyroid hormone.
Trial registration: ClinicalTrials.gov NCT00345839.
Keywords: CKD; cardiovascular disease; hemodialysis; hyperparathyroidism; mineral metabolism.
Copyright © 2015 by the American Society of Nephrology.
Figures


References
-
- Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, Collins AJ: Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 16: 489–495, 2005 - PubMed
-
- Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32[Suppl 3]: S112–S119, 1998 - PubMed
-
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group : KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 76: S1–S130, 2009 - PubMed
-
- London GM, Guérin AP, Marchais SJ, Métivier F, Pannier B, Adda H: Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 18: 1731–1740, 2003 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

