Microcircuits and their interactions in epilepsy: is the focus out of focus?
- PMID: 25710837
- PMCID: PMC4561622
- DOI: 10.1038/nn.3950
Microcircuits and their interactions in epilepsy: is the focus out of focus?
Abstract
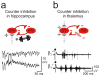
Epileptic seizures represent dysfunctional neural networks dominated by excessive and/or hypersynchronous activity. Recent progress in the field has outlined two concepts regarding mechanisms of seizure generation, or ictogenesis. First, all seizures, even those associated with what have historically been thought of as 'primary generalized' epilepsies, appear to originate in local microcircuits and then propagate from that initial ictogenic zone. Second, seizures propagate through cerebral networks and engage microcircuits in distal nodes, a process that can be weakened or even interrupted by suppressing activity in such nodes. We describe various microcircuit motifs, with a special emphasis on one that has been broadly implicated in several epilepsies: feed-forward inhibition. Furthermore, we discuss how, in the dynamic network in which seizures propagate, focusing on circuit 'choke points' remote from the initiation site might be as important as that of the initial dysfunction, the seizure 'focus'.
Figures





References
-
- Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV. Jasper’s Basic Mechanisms of the Epilepsies. National Center for Biotechnology Information; Bethesda: 2012. - PubMed
-
- Jones EG. Viewpoint: The core and matrix of thalamic organization. Neuroscience. 1998;85:331–345. - PubMed
-
- Douglas RJ, Koch C, Mahowald M, Martin KA, Suarez HH. Recurrent excitation in neocortical circuits. Science. 1995;269:981–985. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

