Oxidized LDL activates blood platelets through CD36/NOX2-mediated inhibition of the cGMP/protein kinase G signaling cascade
- PMID: 25710879
- PMCID: PMC4408294
- DOI: 10.1182/blood-2014-05-574491
Oxidized LDL activates blood platelets through CD36/NOX2-mediated inhibition of the cGMP/protein kinase G signaling cascade
Abstract
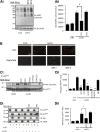
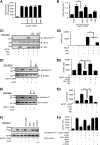
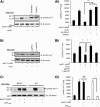
Oxidized low-density lipoprotein (oxLDL) promotes unregulated platelet activation in dyslipidemic disorders. Although oxLDL stimulates activatory signaling, it is unclear how these events drive accelerated thrombosis. Here, we describe a mechanism for oxLDL-mediated platelet hyperactivity that requires generation of reactive oxygen species (ROS). Under arterial flow, oxLDL triggered sustained generation of platelet intracellular ROS, which was blocked by CD36 inhibitors, mimicked by CD36-specific oxidized phospholipids, and ablated in CD36(-/-) murine platelets. oxLDL-induced ROS generation was blocked by the reduced NAD phosphate oxidase 2 (NOX2) inhibitor, gp91ds-tat, and absent in NOX2(-/-) mice. The synthesis of ROS by oxLDL/CD36 required Src-family kinases and protein kinase C (PKC)-dependent phosphorylation and activation of NOX2. In functional assays, oxLDL abolished guanosine 3',5'-cyclic monophosphate (cGMP)-mediated signaling and inhibited platelet aggregation and arrest under flow. This was prevented by either pharmacologic inhibition of NOX2 in human platelets or genetic ablation of NOX2 in murine platelets. Platelets from hyperlipidemic mice were also found to have a diminished sensitivity to cGMP when tested ex vivo, a phenotype that was corrected by infusion of gp91ds-tat into the mice. This study demonstrates that oxLDL and hyperlipidemia stimulate the generation of NOX2-derived ROS through a CD36-PKC pathway and may promote platelet hyperactivity through modulation of cGMP signaling.
© 2015 by The American Society of Hematology.
Figures




Comment in
-
Disabling the platelet's brakes to promote thrombosis.Blood. 2015 Apr 23;125(17):2591-3. doi: 10.1182/blood-2015-03-630822. Blood. 2015. PMID: 25907902
References
-
- Schmidt HH, Lohmann SM, Walter U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Biochim Biophys Acta. 1993;1178(2):153–175. - PubMed
-
- Carvalho AC, Colman RW, Lees RS. Platelet function in hyperlipoproteinemia. N Engl J Med. 1974;290(8):434–438. - PubMed
-
- Davì G, Romano M, Mezzetti A, et al. Increased levels of soluble P-selectin in hypercholesterolemic patients. Circulation. 1998;97(10):953–957. - PubMed
-
- Pawlowska Z, Swiatkowska M, Krzeslowska J, Pawlicki L, Cierniewski CS. Increased platelet-fibrinogen interaction in patients with hypercholesterolemia and hypertriglyceridemia. Atherosclerosis. 1993;103(1):13–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

