Blimp-1, an intrinsic factor that represses HIV-1 proviral transcription in memory CD4+ T cells
- PMID: 25710909
- PMCID: PMC4369419
- DOI: 10.4049/jimmunol.1402581
Blimp-1, an intrinsic factor that represses HIV-1 proviral transcription in memory CD4+ T cells
Abstract
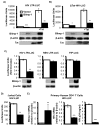
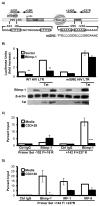
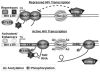
CD4(+) T cell subsets differentially support HIV-1 replication. For example, quiescent CD4(+) memory T cells are susceptible to HIV-1 infection but do not support robust HIV-1 transcription and have been implicated as the primary reservoir of latent HIV-1. T cell transcription factors that regulate maturation potentially limit HIV-1 transcription and mediate the establishment and maintenance of HIV-1 latency. We report that B lymphocyte-induced maturation protein-1 (Blimp-1), a critical regulator of B and T cell differentiation, is highly expressed in memory CD4(+) T cells compared with naive CD4(+) T cells and represses basal and Tat-mediated HIV-1 transcription. Blimp-1 binds an IFN-stimulated response element within HIV-1 provirus, and it is displaced following T cell activation. Reduction of Blimp-1 in infected primary T cells including CD4(+) memory T cells increases RNA polymerase II processivity, histone acetylation, and baseline HIV-1 transcription. Therefore, the transcriptional repressor, Blimp-1, is an intrinsic factor that predisposes CD4(+) memory T cells to latent HIV-1 infection.
Copyright © 2015 by The American Association of Immunologists, Inc.
Figures

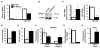

References
-
- Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. - PubMed
-
- Turner CA, Mack DH, Davis MM. Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell. 1994;77:297–306. - PubMed
-
- Lin Y, Wong K, Calame K. Repression of c-myc transcription by Blimp-1, an inducer of terminal B cell differentiation. Science. 1997;276:596–599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

